Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Group 13 (Boron Group) Elements
The boron occurs mostly as borates and its important ores are borax – Na2[B4O5(OH)4].8H2O and kernite – Na2[B4O5(OH)4].2H2O. Aluminium is the most abundant metal and occurs as oxides and also found in aluminosilicate rocks. Commercially it is extracted from its chief ore, bauxite (Al2O3.2H2O). The other elements of this group occur only in trace amounts. The other elements Ga, In and Tl occur as their sulphides.
Physical Properties:
Some of the physical properties of the group 13 elements are listed below
Table 2.3 Physical properties of group 13 elements
|
Element |
Most Common Allotropes |
| Boron | Amorphous boron, α-rhombohedral boron, β-rhombohedral boron, γ-orthorhombic boron, α-tetragonal boron, β-tetragonal boron |
| Carbon | Diamond, Graphite, Graphene, Fullerenes, Carbon nanotubes |
| Silicon | Amorphous Silicon, Crystalline Silicon |
| Germanium | α-germanium, β-germanium |
| Tin | Grey tin, white tin, rhombic tin, sigma tin |
| Phosphorus | White phosphorus, Red phosphorus, Scarlet phosphorus, Violet phosphorus, Black phosphorus. |
| Arsenic | Yellow arsenic, gray arsenic & Black arsenic |
| Anitimony | Blue-white antimony, Yellow, Black |
| Oxygen | Dioxygen, ozone |
| Sulphur | Rhombus sulphur, monoclinic sulphur |
| Selenium | Red selenium, Gray selenium, Black selenium, Monoclinic selenium |
| Tellurium | Amorphous & Crystalline |
Chemical Properties of Boron:
Boron is the only nonmetal in this group and is less reactive. However, it shows reactivity at higher temperatures. Many of its compounds are electron deficient and has unusual type of covalent bonding which is due to its small size, high ionisation energy and similarity in electronegativity with carbon and hydrogen.
Formation of Metal Borides:
Many metals except alkali metals form borides with a general formula MxBy (x ranging upto 11 and y ranging upto 66 or higher) Direct combination of metals with boron:
Direct Combination of Metals With Boron:
![]()
Reduction of Boron Trihalides:
Reduction of borontrichloride with a metal assisted by dihydrogen gives metal borides.
![]()
Formation of Hydrides:
Boron does not react directly with hydrogen. However, it forms a variety of hydrides called boranes. The simplest borane is diborane – B2H6. Other larger boranes can be prepared from diborane. Treatment of gaseous boron triflouride with sodium hydride around 450 K gives diborane. To prevent subsequent pyrolysis, the product diborane is trapped immediately.
![]()
Formation of Boron Trihalides:
Boron combines with halogen to form boron trihalides at high temperatures.
![]()
Formation of Boron Nitride:
Boron burns with dinitrogen at high temperatures to form boron nitride.
![]()
Formation of Oxides:
When boron is heated with oxygen around 900 K, it forms its oxide.
![]()
Reaction with Acids and Alkali:
Halo acids have no reaction with boron. However, boron reacts with oxidising acids such as sulphuric acid and nitric acids and forms boric acid.
2B + 3H2SO4 → 2H3BO3 + 3SO2
B + 3HNO3 → H3BO3 + 3NO2
Boron reacts with fused sodium hydroxide and forms sodium borate.
2B + 6NaOH → 2Na2BO3 + 3H2
Uses of Boron:
- Boron has the capacity to absorb neutrons. Hence, its isotope 10B5 is used as moderator in nuclear reactors.
- Amorphous boron is used as a rocket fuel igniter.
- Boron is essential for the cell walls of plants.
- Compounds of boron have many applications. For example eye drops, antiseptics, washing powders etc contains boric acid and borax. In the manufacture of Pyrex glass, boric oxide is used.
Borax [Na2B4O7.10H2O]:
Preparation:
Borax is a sodium salt of tetraboric acid. It is obtained from colemanite ore by boiling its solution with sodium carbonate.
![]()
Borax is normally formulated as Na2B4O7.10H2O. But it contains, tetranuclear units [B4O5. (OH)4]2-. This form is known as prismatic form. Borax also exists two other forms namely, jeweller or octahderal borax (Na2B4O7.5H2O) and borax glass (Na2B4O7).
Properties
Borax is basic in nature and its solution in hot-water is alkaline as it dissociates into boric acid and sodium hydroxide.
Na2B4O7 + 7H2O → 4H3BO3 + 2NaOH
On heating it forms a transparent borax beads.
![]()
Borax reacts with acids to form sparingly soluble boric acid.
Na2B4O7 + 2HCl + 5H2O → 4H3BO3 + 2NaCl
Na2B4O7 + H2SO4 + 5H2O → 4H3BO3 + Na2SO4
When treated with ammonium chloride it forms boron nitride.
Na2B4O7 + 2NH4Cl → 2NaCl + 2BN + B2O3 + 4H2O
Uses of Borax:
- Borax is used for the identifiation of coloured metal ions
- In the manufacture optical and borosilicate glass, enamels and glazes for pottery
- It is also used as a flx in metallurgy and also acts as a preservative
Boric Acid [H3BO3 or B(OH)3]:
Preparation:
Boric acid can be extracted from borax and colemanite.
Na2B4O7 + H2SO4 → 4H3BO3 + Na2SO4
Ca2B6O11 + 11H2O + 4SO2 → 2Ca(HSO3)2 + 6H3BO3
Properties:
Boric acid is a colourless transparent crystal. It is a very weak monobasic acid and, it accepts hydroxyl ion rather than donating proton.
B(OH)3 + 2H2O ⇄ H3O+ + [B(OH)4]–
It reacts with sodium hydroxide to form sodium metaborate and sodium tetraborate.
H3BO3 + NaOH → NaBO2 + 2H2O
4H3BO3 + 2NaOH → Na2B4O7 + 7H2O
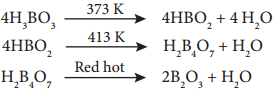
Action of Heat:
Boric acid when heated at 373 K gives metaboric acid and at 413 K, it gives tetraboric acid. When heated at red hot, it gives boric anhydride which is a glassy mass.
Action of Ammonia
Fusion of urea with B(OH)3, in an atmosphere of ammonia at 800 – 1200 K gives boron nitride.
![]()
Ethyl Borate Test
When boric acid or borate salt is heated with ethyl alcohol in presence of conc. sulphuric acid, an ester, triethylborate is formed. The vapour of this ester burns with a green edged flame and this reaction is used to identify the presence of borate.
![]()
Note:
The trialkyl borate on reaction with sodium hydride in tetrahydrofuran to form a coordination compound Na[BH(OR)3], which acts as a powerful reducing agent.
Formation of Boron Triflouride:
Boric acid reacts with calcium flouride in presence of conc. sulphuric acid and gives boron triflouride.
3CaF2 + 3H2SO4 + 2 B(OH3) → 3CaSO4 + 2BF3 + 6H2O
Boric acid when heated with soda ash it gives borax
Na2CO3 + 4B(OH)3 → Na2B4O7 + CO2 + 6H2O
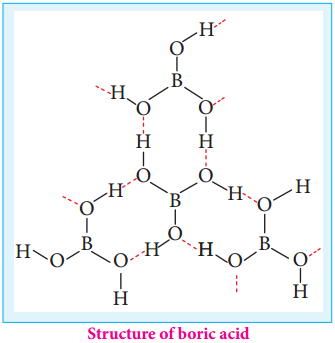
Structure of Boric Acid:
Boric acid has a two dimensional layered structure. It consists of [BO3]3- unit and these are linked to each other by hydrogen bonds as shown in the Figure 2.2.
Uses of Boric Acid:
- Boric acid is used in the manufacture of pottery glases, enamels and pigments.
- It is used as an antiseptic and as an eye lotion.
- It is also used as a food preservative.
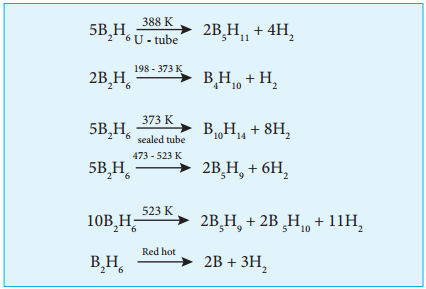
Diborane
Preparation:
As discussed earlier diborane can be prepared by the action of metal hydride with boron. This method is used for the industrial production. Diborane can also be obtained in small quantities by the reaction of iodine with sodium borohydride in diglyme.
2NaBH4 + I2 → B2H6 + 2NaI + H2
On heating magnesium boride with HCl a mixture of volatile boranes are obtained.
2Mg3B2 + 12HCl → 6MgCl2 + B4H10 + H2
B4H10 + H2 → 2B2H6
Properties:
Boranes are colourless diamagnetic compounds with low thermal stability. Diborane is a gas at room temperature with sweet smell and it is extremely toxic. It is also highly reactive.
At high temperatures it forms higher boranes liberating hydrogen.
Diboranes reacts with water and alkali to give boric acid and metaborates respectively.
B2H6 + 6H2O → 2H3BO3 + 6H2
B2H6 + 2NaOH + 2H2O → 2NaBO2 + 6H2
Action of Air:
At room temperature pure diborane does not react with air or oxygen but in impure form it gives B2O3 along
with large amount of heat.
B2H6 + 3O2 → B2O3 + 3H2O
∆H = – 2165 KJ mol2-
Diborane reacts with methyl alcohol to give trimethyl Borate.
B2H6 + 6CH3OH → 2B(OCH3)3 + 6H2
Hydroboration:
Diborane adds on to alkenes and alkynes in ether solvent at room temperature. This reaction is called hydroboration and is highly used in synthetic organic chemistry, especially for anti Markovnikov addition.
B2H6 + 6RCH = CHR → 2(RCH2 – CHR)3B
Reaction with Ionic Hydrides
When treated with metal hydrides it forms metal borohydrides

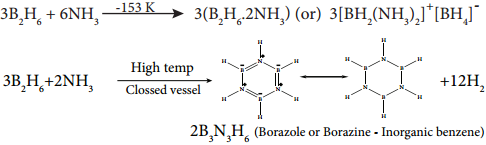
Reaction with Ammonia:
When treated with excess ammonia at low temperatures diborane gives diboranediammonate. On heating at higher temperatures it gives borazole.
Structure of Diborane:
In diborane two BH2 units are linked by two bridged hydrogens. Therefore, it has eight B-H bonds. However, diborane has only 12 valance electrons and are not sufficient to form normal covalent bonds. The four terminal B-H bonds are normal covalent bonds (two centre – two electron bond or 2c-2e bond).
The remaining four electrons have to be used for the bridged bonds. i.e. two three centred B-H-B bonds utilise two electrons each. Hence, these bonds are three centre-two electron bonds (3c-2e). The bridging hydrogen atoms are in a plane as shown in the figure 2.3. In diborane, the boron is sp3 hybridised.
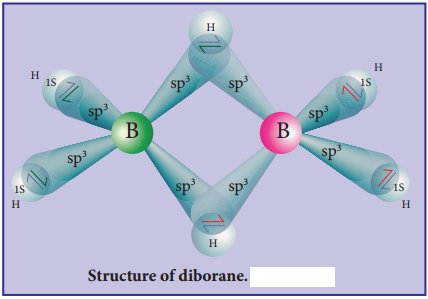
Three of the four sp3 hybridised orbitals contains single electron and the fourth orbital is empty. Two of the half filled hybridised orbitals of each boron overlap with the 1s orbitals of two hydrogens to form four terminal 2c-2e bonds, leaving one empty and one half filled hybridised orbitals on each boron. The Three centre – two electron bonds), B-H-B bond formation involves overlapping the half filled hybridised orbital of one boron, the empty hybridised orbital of the other boron and the half filled 1s orbital of hydrogen.
Uses of Diborane:
- Diborane is used as a high energy fuel for propellant
- It is used as a reducing agent in organic chemistry
- It is used in welding torches
Boron Triflouride:
Preparation:
Boron triflouride is obtained by the treatment of calcium fluoride with boron trioxide in presence of conc. sulphuric acid.
![]()
It can also be obtained by treating boron trioxide with carbon and fluorine.
B2O3 + 3C + 3F2 → 2BF3 + 3CO
In the laboratory pure BF3 is prepared by the thermal decomposition of benzene diazonium tetrafloro borate.
![]()
Properties:
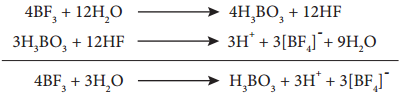
Boron triflouride has a planar geometry. It is an electron deficient compound and accepts electron pairs to form coordinate covalent bonds. They form complex of the type [BX4]2-.
BF3 + NH3 → F3B ← NH3
BF3 + H2O → F3B ← OH3
On hydrolysis, boric acid is obtained. This then gets converted into Hydro floroboric acid.
Uses of Boron Triflouride:
- Boron trifloride is used for preparing HBF4, a catalyst in organic chemistry
- It is also used as a flourinating reagent.
Aluminium Chloride:
Preparation:
When aluminium metal or aluminium hydroxide is treated with hydrochloric acid, aluminium trichloride is formed. The reaction mixture is evaporated to obtain hydrated aluminium chloride.
2Al + 6HCl → 2AlCl3 + 3H2
Al(OH)3 + 3HCl → AlCl3 + 3H2O
McAfee Process:
Aluminium chloride is obtained by heating a mixture of alumina and coke in a current of chlorine.
2Al2O3 + 3C + 6Cl2 → 4AlCl3 + 3CO2
On industrial scale it is prepared by chlorinating aluminium around 1000 K
![]()
Properties:
Anhydrous aluminium chloride is a colourless, hygroscopic substance. An aqueous solution of aluminium chloride is acidic in nature. It also produces hydrogen chloride fumes in moist air.
AlCl3 + 3H2O → Al(OH)3 + 3HCl
With ammonium hydroxide it forms aluminium hydroxide.
AlCl3 + 3NH4OH → Al(OH)3 + 3NH4Cl
With excess of sodium hydroxide it produces metal aluminate
AlCl3 + 4NaOH → NaAlO2 + 2H2O + 3NaCl
It behaves like a Lewis acid and forms addition compounds with ammonia, phosphine and carbonylchloride etc… Eg. AlCl3.6NH3.
Uses of Aluminium Chloride:
- Anhydrous aluminium chloride is used as a catalyst in Friedels Craft reactions
- It is used for the manufacture of petrol by cracking the mineral oils.
- It is used as a catalyst in the manufacture on dyes, drugs and perfumes.
Alums:
The name alum is given to the double salt of potassium aluminium sulphate [K2SO4.Al2(SO4)3.24.H2O]. Now a days it is used for all the double salts with M‘SO4.M”2(SO4)3. 24H2O, where M’ is univalent metal ion or [NH4]+ and M” is trivalent metal ion.
Examples:
Potash alum [K2SO4.Al2(SO4)3.24.H2O]; Sodium alum [Na2SO4.Al2(SO4)3. 24.H2O], Ammonium alum [(NH4)2(SO4)3.24.H2O], Chrome alum [K2SO4.Cr2(SO4)3.24.H2O]. Alums in general are more soluble in hot water than in cold water and in solutions they exhibit the properties of constituent ions.
Preparation:
The alunite the alum stone is the naturally occurring form and it is K2SO4. Al2(SO4)3. 4Al(OH)3. When alum stone is treated with excess of sulphuric acid, the aluminium hydroxide is converted to aluminium sulphate. A calculated quantity of potassium sulphate is added and the solution is crystallised to generate potash alum. It is purified by recrystallisation.
K2SO4.Al2(SO4)3.4Al(OH)3 + 6H2SO4 → K2SO4 + 3Al2(SO4)3 + 12H2O
K2SO4 + Al2(SO4)3 + 24 H2O → K2SO4.Al2(SO4)3. 24 H2O
Properties
Potash alum is a white crystalline solid it is soluble in water and insoluble in alcohol. The aqueous solution is acidic due to the hydrolysis of aluminium sulphate it melts at 365 K on heating. At 475 K loses water of hydration and swells up. The swollen mass is known as burnt alum. Heating to red hot it decomposes into potassium sulphate, alumina and sulphur trioxide.
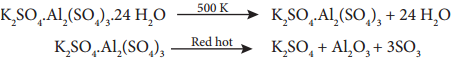
Potash alum forms aluminium hydroxide when treated with ammonium hydroxide.
K2SO4.Al2(SO4)3.24 H2O + 6NH4OH → K2SO4 + 3(NH4)2SO4 + 24 H2O + 2Al(OH)3
Uses of Alum:
- It is used for purification of water
- It is also used for water proofing and textiles
- It is used in dyeing, paper and leather tanning industries
- It is employed as a styptic agent to arrest bleeding.