Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Group 14 (Carbon Group) Elements
Occurrence:
Carbon is found in the native form as graphite. Coal, crude oil and carbonate rocks such as calcite, magnesite etc contains large quantities of carbon in its combined form with other elements. Silicon occurs as silica (sand and quartz crystal). Silicate minerals and clay are other important sources for silicon.
Physical Properties:
Some of the physical properties of the group 14 elements are listed below
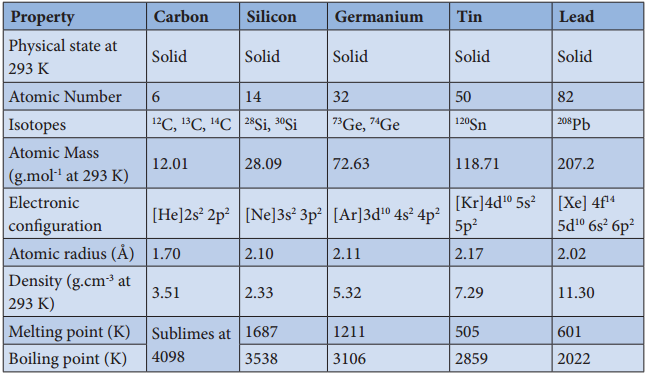
Table 2.4 Physical properties of group 14 elements
Tendency for Catenation
Catenation is an ability of an element to form chain of atoms. The following conditions are necessary for catenation.
- The valency of element is greater than or equal to two
- Element should have an ability to bond with itself
- The self bond must be as strong as its bond with other elements
- Kinetic inertness of catenated compound towards other molecules.
Carbon possesses all the above properties and forms a wide range of compounds with itself and with other elements such as H, O, N, S and halogens.
Allotropes of Carbon
Carbon exists in many allotropic forms. Graphite and diamond are the most common allotropes. Other important allotropes are graphene, fullerenes and carbon nanotubes.
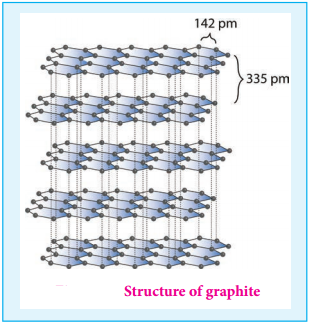
Graphite is the most stable allotropic form of carbon at normal temperature and pressure. It is soft and conducts electricity. It is composed of flat two dimensional sheets of carbon atoms. Each sheet is a hexagonal net of sp2 hybridised carbon atoms with a C-C bond length of 1.41 Å which is close to the C-C bond distance in benzene (1.40 Å). Each carbon atom forms three σ bonds with three neighbouring carbon atoms using three of its valence electrons and the fourth electron present in the unhybridised p orbital forms a π-bond.
These π electrons are delocalised over the entire sheet which is responsible for its electrical conductivity. The successive carbon sheets are held together by weak vander Waals forces. The distance between successive sheet is 3.40 Å. It is used as a lubricant either on its own or as a graphited oil.
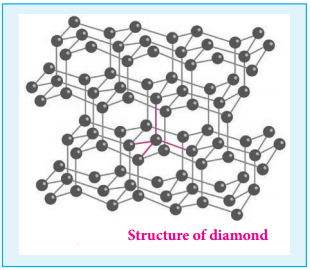
Unlike graphite the other allotrope diamond is very hard. The carbon atoms in diamond are sp3 hybridised and bonded to four neighbouring carbon atoms by σ bonds with a C-C bond length of 1.54 Å. This results in a tetrahedral arrangement around each carbon atom that extends to the entire lattice as shown in figure 2.5. Since all four valance electrons of carbon are involved in bonding there is no free electrons for conductivity. Being the hardest element, it used for sharpening hard tools, cutting glasses, making bores and rock drilling.
Fullerenes are newly synthesised allotropes of carbon. Unlike graphite and diamond, these allotropes are discrete molecules such as C32, C50, C60, C70, C76 etc. These molecules have cage like structures as shown in the figure. The C60 molecules have a soccer ball like structure and is called buckminster fullerene or buckyballs.
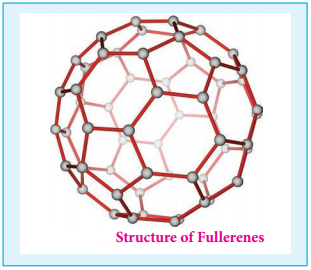
It has a fused ring structure consists of 20 six membered rings and 12 five membered rings. Each carbon atom is sp2 hybridised and forms three σ bonds & a delocalised π bond giving aromatic character to these molecules. The C-C bond distance is 1.44 Å and C = C distance 1.38 Å.
Carbon nanotubes, another recently discovered allotropes, have graphite like tubes with fullerene ends. Along the axis, these nanotubes are stronger than steel and conduct electricity. These have many applications in nanoscale electronics, catalysis, polymers and medicine.
Another allotrophic form of carbon is graphene. It has a single planar sheet of sp2 hybridised carbon atoms that are densely packed in a honeycomb crystal lattice.
Carbon Monoxide [CO]:
Preparation:
Carbon monoxide can be prepared by the reaction of carbon with limited amount of oxygen.
2C + O2 → 2CO
On industrial scale carbon monoxide is produced by the reaction of carbon with air. The carbon monoxide formed will contain nitrogen gas also and the mixture of nitrogen and carbon monoxide is called producer gas.
The producer gas is then passed through a solution of copper(I)chloride under pressure which results in the formation of CuCl(CO).2H2O. At reduced pressures this solution releases the pure carbon monoxide. Pure carbon monoxide is prepared by warming methanoic acid with concentrated sulphuric acid which acts as a dehydrating agent.
HCOOH + H2SO4 → CO + H2SO4.H2O
Properties
- It is a colourless, odourless, and poisonous gas. It is slightly soluble in water.
- It burns in air with a blue flame forming carbon dioxide.
2CO + O2 → 2CO2
When carbon monoxide is treated with chlorine in presence of light or charcoal, it forms a poisonous gas carbonyl chloride, which is also known as phosgene. It is used in the synthesis of isocyanates.
CO + Cl2 → COCl2
Carbon monoxide acts as a strong reducing agent.
3CO + Fe2 O3 → 2Fe + 3CO2
Under high temperature and pressure a mixture of carbon monoxide and hydrogen (synthetic gas or syn gas) gives methanol.
CO + 2H2 → CH3OH
In oxo process, ethene is mixed with carbon monoxide and hydrogen gas to produce propanal.
CO + C2H4 + H4 → CH3CH2CHO
Fischer Tropsch Synthesis:
The reaction of carbon monoxide with hydrogen at a pressure of less than 50 atm using metal catalysts at 500 – 700 K yields saturated and unsaturated hydrocarbons.
nCO + (2n+1)H2 → CnH(2n+2) + nH2O
nCO + 2nH2 → CnH2n + nH2O
Carbon monoxide forms numerous complex compounds with transition metals in which the transition meal is in zero oxidation state. These compounds are obtained by heating the metal with carbon monoxide.
Eg. Nickel tetracarbonyl [Ni(CO)4], Iron pentacarbonyl [Fe(CO)5], Chromium hexacarbonyl [Cr(CO)6].
Structure:
It has a linear structure. In carbon monoxide, three electron pairs are shared between carbon and oxygen. The bonding can be explained using molecular orbital theory as discussed in XI standard. The C-O bond distance is 1.128Å. The structure can be considered as the resonance hybrid of the following two canonical forms.
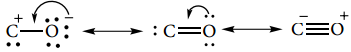
Uses of Carbon Monoxide:
- Equimolar mixture of hydrogen and carbon monoxide – water gas and the mixture of carbon monoxide and nitrogen – producer gas are important industrial fuels.
- Carbon monoxide is a good reducing agent and can reduce many metal oxides to metals.
- Carbon monoixde is an important ligand and forms carbonyl compound with transition metals.
Carbon Dioxide:
Carbon dioxide occurs in nature in free state as well as in the combined state. It is a constituent of air (0.03%). It occurs in rock as calcium carbonate and magnesium carbonate.
Production
On industrial scale it is produced by burning coke in excess of air.
C + O2 → CO2 ∆H = – 394 kJ mol-1
Calcination of lime produces carbon dioxide as by product.
CaCO3 → CaO + CO2
Carbon dioxide is prepared in laboratory by the action of dilute hydrochloric acid on metal carbonates.
CaCO3 + 2HCl → CaCl2 + H2O + CO2
Properties
It is a colourless, nonflammable gas and is heavier than air. Its critical temperature is 31° C and can be readily liquefied.
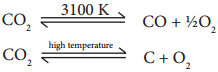
Carbon dioxide is a very stable compound. Even at 3100 K only 76% decomposes to form carbon monoxide and oxygen. At still higher temperature it decomposes into carbon and oxygen.
Oxidising Behaviour:
At elevated temperatures, it acts as a strong oxidising agent. For example,
CO2 + 2Mg → 2MgO + C
Water Gas Equilibrium:
The equilibrium involved in the reaction between carbon dioxide and hydrogen, has many industrial applications and is called water gas equilibrium.
![]()
Acidic Behaviour:
The aqueous solution of carbon dioxide is slightly acidic as it forms carbonic acid.
CO2 + H2O ⇄ H2CO3 ⇄ H+ + HCO3–
Structure of Carbon Dioxide
Carbon dioxide has a linear structure with equal bond distance for the both C-O bonds. In this molecule there is two C-O sigma bond. In addition there is 3c-4e bond covering all the three atoms.
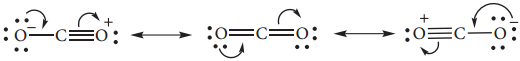
Uses of Carbon Dioxide
- Carbon dioxide is used to produce an inert atomosphere for chemical processing.
- Biologically, it is important for photosynthesis.
- It is also used as fie extinguisher and as a propellent gas.
- It is used in the production of carbonated beverages and in the production of foam.
Silicon Tetrachloride:
Preparation:
Silicon tetrachloride can be prepared by passing dry chlorine over an intimate mixture of silica and carbon by heating to 1675 K in a porcelain tube
SiO2 + 2C + 2Cl2 → SiCl4 + 2CO
On commercial scale, reaction of silicon with hydrogen chloride gas occurs above 600 K
Si + 4HCl → SiCl4 + 2H2
Properties:
Silicon tetrachloride is a colourless fuming liquid and it freezes at – 70°C. In moist air, silicon tetrachloride is hydrolysed with water to give silica and hydrochloric acid.
SiCl4 + 2H2O → 4HCl + SiO2
When silicon tetrachloride is hydrolysed with moist ether, linear perchloro siloxanes are formed [Cl-(Si Cl2O)nSiCl3 where n = 1 – 6.
Alcoholysis
The chloride ion in silicon tetrachloride can be substituted by nucleophile such as OH, OR, etc using suitable reagents. For example, it forms silicic esters with alcohols.
![]()
Ammonialysis
Similarly silicon tetrachloride undergoes ammonialysis to form chlorosilazanes.
![]()
Uses:
- Silicon tetrachloride is used in the production of semiconducting silicon.
- It is used as a starting material in the synthesis of silica gel, silicic esters, a binder for ceramic materials.
Silcones:
Silicones or poly siloxanes are organo silicon polymers with general empirical formula (R2SiO). Since their empirical formula is similar to that of ketone (R2CO), they were named “silicones”. These silicones may be linear or cross linked. Because of their very high thermal stability they are called high – temperature polymers.
Preparation:
Generally silicones are prepared by the hydrolysis of dialkyldichlorosilanes (R2SiCl2) or diaryldichlorosilanes
Ar2SiCl2, which are prepared by passing vapours of RCl or ArCl over silicon at 570 K with copper as a catalyst.
![]()
The hydrolysis of dialkylchloro silanes R2SiCl2 yields to a straight chain polymer which grown from both the sides
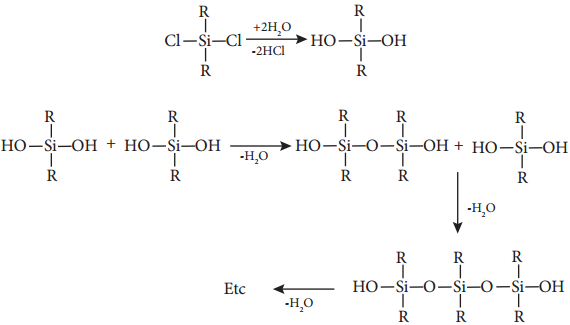
The hydrolysis of monoalkylchloro silanes RSiCl3 yields to a very complex cross linked polymer. Linear silicones can be converted into cyclic or ring silicones when water molecules is removed from the terminal – OH groups.
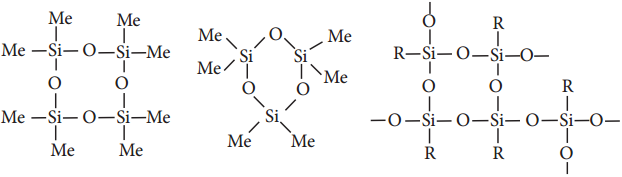
Types of Silicones:
(i) Liner Silicones:
They are obtained by the hydrolysis and subsequent condensation of dialkyl or diaryl silicon chlorides.
(a) Silicone Rubbers:
These silicones are bridged together by methylene or similar groups.
(b) Silicone Resins:
They are obtained by blending silicones with organic resins such as acrylic esters.
(ii) Cyclic Silicones
These are obtained by the hydrolysis of R2SiCl2.
(iii) Cross linked Silicones
They are obtained by hydrolysis of RSiCl3
Properties
The extent of cross linking and nature of alkyl group determine the nature of polymer. They range from oily liquids to rubber like solids. All silicones are water repellent. This property arises due to the presence of organic side groups that surrounds the silicon which makes the molecule looks like an alkane.
They are also thermal and electrical insulators. Chemically they are inert. Lower silicones are oily liquids whereas higher silicones with long chain structure are waxy solids. The viscosity of silicon oil remains constant and doesn’t change with temperature and they don’t thicken during winter.
Uses:
- Silicones are used for low temperature lubrication and in vacuum pumps, high temperature oil baths etc
- They are used for making water proofing clothes
- They are used as insulting material in electrical motor and other appliances
- They are mixed with paints and enamels to make them resistant towards high temperature, sunlight, dampness and chemicals.
Silicates
The mineral which contains silicon and oxygen in tetrahedral [SiO4]2- units linked together in different patterns are called silicates. Nearly 95% of the earth crust is composed of silicate minerals and silica. The glass and ceramic industries are based on the chemistry silicates.
Types of Silicates:
Silicates are classified into various types based on the way in which the tetrahedral units, [SiO4]4- are
linked together.
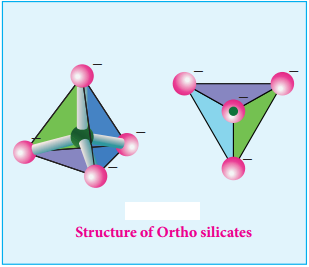
Ortho Silicates (Neso Silicates):
The simplest silicates which contain discrete [SiO4]4- tetrahedral units are called ortho silicates or
neso silicates.
Examples:
Phenacite – Be2SiO4 (Be2+ ions are tetrahedrally surrounded by O2- ions), Olivine – (Fe/Mg)2SiO4 (Fe2+ and Mg2+ cations are octahedrally surrounded by O2- ions).
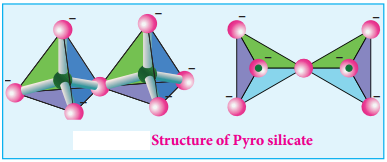
Pyro Silicate (or) Soro Silicates:
Silicates which contain [Si2O7]6- ions are called pyro silicates (or) Soro silicates. They are formed by joining two [SiO4]4- tetrahedral units by sharing one oxygen atom at one corner (one oxygen is removed while joining). Example: Thortveitite – Sc2Si2O7
Cyclic Silicates (or Ring Silicates)
Silicates which contain (SiO3)n2n- which are formed by linking three or more tetrahedral SiO42- units cyclically are called cyclic silicates. Each silicate unit shares two of its oxygen atoms with other units.
Example: Beryl [Be3Al2 (SiO3)6] (an aluminosilicate with each aluminium is surrounded by 6 oxygen atoms octahedrally)
Inosilicates:
Silicates which contain ‘n’ number of silicate units linked by sharing two or more oxygen atoms are called inosilicates. They are further classified as chain silicates and double chain silicates.
Chain Silicates (or Pyroxenes):
These silicates contain [(SiO3)n]2n- ions formed by linking ‘n’ number of tetrahedral [SiO4]4- units linearly. Each silicate unit shares two of its oxygen atoms with other units. Example: Spodumene – LiAl(SiO3)2.
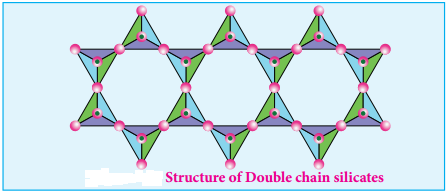
Double Chain Silicates (or Amphiboles):
These silicates contains [Si4O11]n6n- ions. In these silicates there are two different types of tetrahedra:
- These sharing 3 vertices
- Those sharing only 2 vertices.
Examples:
1. Asbestos:
These are fibrous and noncombustible silicates. Therefore they are used for thermal insulation material, brake linings, construction material and filters. Asbestos being carcinogenic silicates, their applications are restricted.
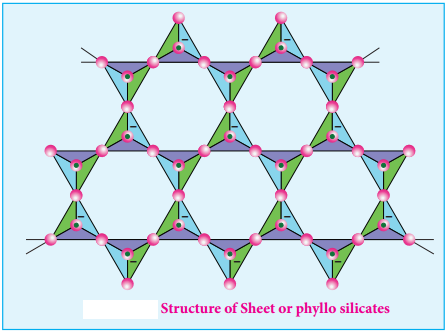
Sheet or Phyllo Silicates
Silicates which contain (Si2O5)n2n- are called sheet or phyllo silicates. In these, Each [SiO4]4- tetrahedron unit shares three oxygen atoms with others and thus by forming twodimensional sheets. These sheets silicates form layered structures in which silicate sheets are stacked over each other. The attractive forces between these layers are very weak, hence they can be cleaved easily just like graphite.
Example: Talc, Mica etc.
Three Dimensional Silicates (or Tecto Silicates):
Silicates in which all the oxygen atoms of [SiO4]2- tetrahedra are shared with other tetrahedra to form three-dimensional network are called three dimensional or tecto silicates. They have general formula (SiO2)n.
Examples: Quartz
These tecto silicates can be converted into three dimensional aluminosilicates by replacing [SiO4]4- units
by [AlO4]5- units. Eg. Feldspar, Zeolites etc.,
Zeolites:
Zeolites are three-dimensional crystalline solids containing aluminium, silicon, and oxygen in their regular three dimensional framework. They are hydrated sodium alumino silicates with general formula Na2O.(Al2O3).x(SiO2).yH2O (x=2 to 10; y=2 to 6).
Zeolites have porous structure in which the monovalent sodium ions and water molecules are loosely held. The Si and Al atoms are tetrahedrally coordinated with each other through shared oxygen atoms. Zeolites are similar to clay minerals but they differ in their crystalline structure.
Zeolites have a three dimensional crystalline structure looks like a honeycomb consisting of a network of interconnected tunnels and cages. Water molecules moves freely in and out of these pores but the zeolite framework remains rigid.
Another special aspect of this structure is that the pore/channel sizes are nearly uniform, allowing the crystal to act as a molecular sieve. We have already discussed in XI standard, the removal of permanent hardness of water using zeolites.