Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Group 18 (Inert gases) Elements
Occurrence:
All the noble gases occur in the atmosphere.
Physical Properties:
As we move along the noble gas elements, their atomic radius and boiling point increases from helium to radon. The first ionization energy decreases from helium to radon. Noble gases have the largest ionisation energy compared to any other elements in a given row as they have completely filled orbital in their outer most shell. They are extremely stable and have a small tendency to gain or lose electrons. The common physical properties of the group 18 elements are listed in the Table.
Physical Properties of group 18 Elements
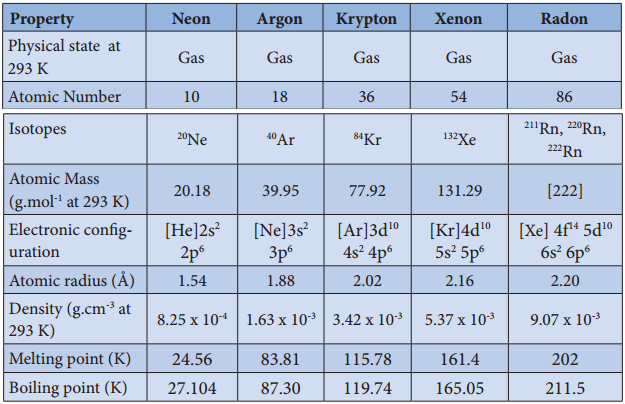
Properties of Inert Gases:
Physical Properties:
Noble gases are monoatomic, odourless, colourless, tasteless, and non-inflammable. They are highly unreactive. They are non-metallic in nature.
Chemical Properties:
Only the xenon and krypton show some chemical reactivity. Xenon fluorides are prepared by direct reaction of xenon and fluorine under different conditions as shown below.

When XeF6 is heated at 50°C in a sealed quartz vessel it forms XeOF4.
![]()
When the reaction is continued the following reaction takes place.
2XeOF4 + SiO2 → 2XeO2F2 + SiF4
2XeO2F2 + SiO2 → 2XeO3 + SiF4
On hydrolysis with water vapour XeF6 gives XeO3
XeF6 + 3H2O → XeO3 + 6HF
When XeF6 reacts with 2.5 M NaOH, sodium per xenate is obtained.
2XeF6 + 16NaOH → Na4XeO6 + Xe + O2 + 12NaF + 8H2O
Sodium per xenate is very much known for its strong oxidizing property. For example, it oxidises manganese (II) ion into permanganate ion even in the absence of the catalyst.
5XeO64- + 2Mn2+ + 14H+ → 2MnO4– + 5XeO3 + 7H2O
Xenon reacts with PtF6 and gave an orange yellow solid [XePtF6] and this is insoluble in CCl4.
Xenon difluoride forms addition compounds XeF2.2SbF5 and XeF2.2TaF5. Xenon
hexa fluorides forms compound with boron and alkali metals. Eg: XeF6.BF3, XeF6MF, M-alkali metals.
There is some evidence for existence of xenon dichloride XeCl2.
Krypton form krypton difluoride when an electric discharge is passed through Kr and flourine at 183°C or when gases are irradiated with SbF5 it forms KrF2.2SbF3.
Structures of Compounds of Xenon:
|
Compound |
Hybridaisation |
Shape/Structure |
| XeF | sp3d | Linear |
| XeF4 | sp3d2 | Square planar |
| XeF6 | sp3d3 | Distorted octahedron |
| XeOF2 | sp3d | T Shaped |
| XeOF4 | sp3d2 | Square pyramidal |
| XeO3 | sp3 | Pyramidal |
Uses of Noble Gases:
The inertness of noble gases is an important feature of their practical uses.
Helium:
- Helium and oxygen mixture is used by divers in place of air oxygen mixture. This prevents the painful dangerous condition called bends.
- Helium is used to provide inert atmosphere in electric arc welding of metals.
- Helium has lowest boiling point hence used in cryogenics (low temperature science)
- It is much less denser than air and hence used for filing air balloons.
Neon:
Neon is used in advertisement as neon sign and the brilliant red glow is caused by passing electric current through neon gas under low pressure.
Argon:
Argon prevents the oxidation of hot filament and prolongs the life in filament bulbs
Krypton:
Krypton is used in florescent bulbs, flash bulbs etc. Lamps filed with krypton are used in airports as approaching lights as they can penetrate through dense fog.
Xenon:
Xenon is used in florescent bulbs, flash bulbs and lasers. Xenon emits an intense light in discharge tubes instantly. Due to this it is used in high speed electronic flash bulbs used by photographers.
Radon:
Radon is radioactive and used as a source of gamma rays. Radon gas is sealed as small capsules and implanted in the body to destroy malignant i.e. cancer growth.