Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Grouping of Elements Based on Electronic Configurations
In the modern periodic table, the elements are organised in 7 periods and 18 groups based on the modern periodic law. The placement of element in the periodic table is closely related to its outer shell electronic configuration. Let us analyse the change in the electronic configuration of elements along the periods and down the groups.
Variation of Electronic Configuration Along the Periods
We have already learnt that each period starts with the element having general outer electronic configuration ns1 and ends with ns2, np6 where n is the period number. The first period starts with the filling of valence electrons in 1s orbital, which can accommodate only two electrons.
Hence, the first period has two elements, namely hydrogen and helium. The second period starts with the filling of valence electrons in 2s orbital followed by three 2p orbitals with eight elements from lithium to neon. The third period starts with filling of valence electrons in the 3s orbital followed by 3p orbitals.
The fourth period starts with filling of valence electrons from 4s orbital followed by 3d and 4p orbitals in accordance with Aufbau principle. Similarly, we can explain the electronic configuration of elements in the subsequent periods (Table 3.10).
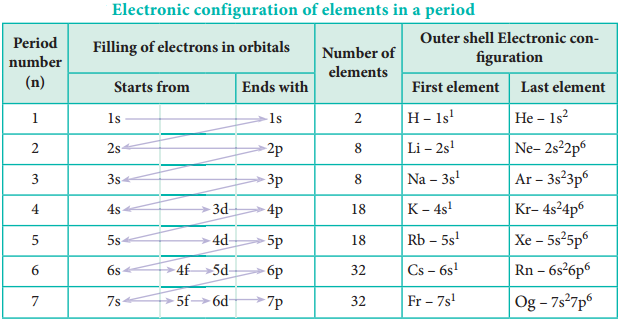
In the fourth period the filling of 3d orbitals starts with scandium and ends with zinc. These 10 elements are called first transition series. Similarly 4d, 5d and 6d orbitals are filled in successive periods and the corresponding series of elements are called second, third and fourth transition series respectively.
In the sixth period the filling of valence electrons starts with 6s orbital followed by 4f, 5d and 6p orbitals. The filling up of 4f orbitals begins with Cerium (Z=58) and ends at Lutetium (Z=71). These 14 elements constitute the first inner-transition series called Lanthanides.
Similarly, in the seventh period 5f orbitals are filled, and it’s -14 elements constitute the second inner transition series called Actinides. These two series are placed separately at the bottom of the modern periodic table.
Variation of Electronic Configuration in the Groups:
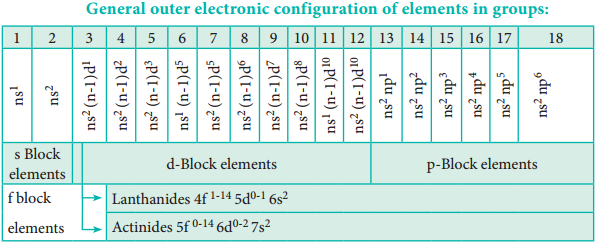
Elements of a group have similar electronic configuration in the outer shell. The general outer electronic configurations for the 18 groups are listed in the Table 3.11. The groups can be combined as s, p, d and f block elements on the basis of the orbital in which the last valence electron enters.
The elements of group 1 and group 2 are called s-block elements, since the last valence electron enters the ns orbital. The group 1 elements are called alkali metals while the group 2 elements are called alkaline earth metals.
These are soft metals and possess low melting and boiling points with low ionisation enthalpies. They are highly reactive and form ionic compounds. They are highly electropositive in nature and most of the elements imparts colour to the flame. We will study the properties of these group elements in detail in subsequent chapters.
The elements of groups 13 to 18 are called p-block elements or representative elements and have a general electronic configuration ns2, np1-6. The elements of the group 16 and 17 are called chalcogens and halogens respectively.
The elements of 18th group contain completely filled valence shell electronic configuration (ns2, np6) and are called inert gases or nobles gases. The elements of p-block have high negative electron gain enthalpies. The ionisation energies are higher than that of s-block elements. They form mostly covalent compounds and shows more than one oxidation states in their compounds.
The elements of the groups 3 to 12 are called d-block elements or transition elements with general valence shell electronic configuration ns1-2, (n-1)d1-10. These elements also show more than one oxidation state and form ionic, covalent and co-ordination compounds. They can form interstitial compounds and alloys which can also act as catalysts. These elements have high melting points and are good conductors of heat and electricity.
The lanthanides (4f1-14, 5d0-1, 6s2) and the actinides (5f0-14, 6d0-2, 7s2) are called f-block elements. These elements are metallic in nature and have high melting points. Their compounds are mostly coloured. These elements also show variable oxidation states.