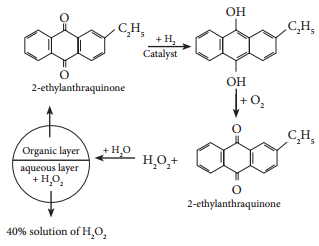
Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Heavy Water of Hydrolysis
Heavy water (D2O) is the oxide of heavy hydrogen. One part of heavy water is present in 5000 parts of ordinary water. It is mainly obtained as the product of electrolysis of water, as D2O does not undergo electrolysis as easily as H2O.
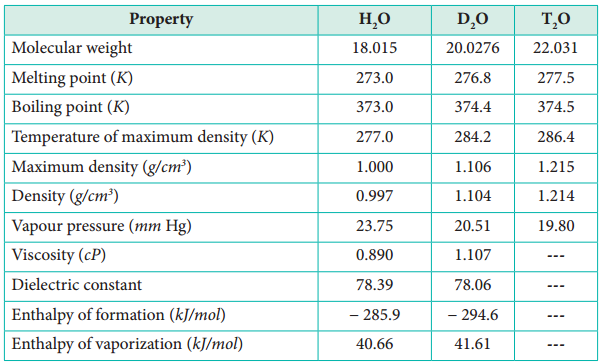
D2O is a colorless, odorless and tasteless liquid. However, there is a marked difference between physical properties of water and heavy water as shown in Table 4.2.
Table: Properties of water, heavy water and super heavy water.
2 NaOH + D2O → 2NaOD + HOD
HCl + D2O → DCl + HOD
NH4Cl + 4D2O → ND4Cl + 4HOD
These exchange reactions are useful in determining the number of ionic hydrogens present in a given compound. For example, when D2O is treated with of hypo-phosphorus acid only one hydrogen atom is exchanged with deuterium. It indicates that, it is a monobasic acid.
H3PO2 + D2O → H2DPO2 + HDO
It is also used to prepare some deuterium compounds:
Al4C3 + 12D2O → 4Al(OD)3 + 3CD4
CaC2 + 2 D2O → Ca(OD)2 + C2D2
Mg3N2 + 6D2O → 3Mg(OD)2 + 2ND3
Ca3P2 + 6D2O → 3Ca(OD)2 + 2PD3
Uses of Heavy Water:
- Heavy water is widely used as moderator in nuclear reactors as it can lower the energies of fast neutrons
- It is commonly used as a tracer to study organic reaction mechanisms and mechanism of metabolic reactions
- It is also used as a coolant in nuclear reactors as it absorbs the heat generated
The heavy water produced is used as a moderator of neutrons in nuclear power plants. In the laboratory heavy water is employed as an isotopic tracer in studies of chemical and biochemical processes.
Heavy water is a form of water with a unique atomic structure and properties coveted for the production of nuclear power and weapons. Like ordinary water-H2O-each molecule of heavy water contains two hydrogen atoms and one oxygen atom. The difference, though, lies in the hydrogen atoms.
The hydrogen is then liquefied and distilled to separate the two components, then the deuterium is reacted with oxygen to form heavy water. Producing heavy water requires advanced infrastructure, and heavy water is actively produced in Argentina, Canada, India, and Norway.
The heavy water is not manufactured, but rather it is extracted from the quantity that is found naturally in lake water. The water is separated through a series of towers, using hydrogen sulphide as an agent.
It was accepted by Norsk Hydro, and production began in 1935. The technology is straightforward. Heavy water (D2O) is separated from normal water by electrolysis, because the difference in mass between the two hydrogen isotopes translates into a slight difference in the speed at which the reaction proceeds.
Heavy water is indeed heavier than normal water (which contains a tiny amount of heavy water molecules naturally), and heavy-water ice will sink in normal water.
Since the chemical properties of the heavier hydrogen-nucleus-with-a-neutron are slightly different, heavy water starts to gum up all manner of body parts. Eventually, if you drank enough purified heavy water-more than 20 gallons, at least a quarter heavy-you’d die.