Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Important Compound of Transition Elements
Oxides and Oxoanions of Metals
Generally, transition metal oxides are formed by the reaction of transition metals with molecular oxygen at high temperatures. Except the first member of 3d series, Scandium, all other transition elements form ionic metal oxides. The oxidation number of metal in metal oxides ranges from +2 to +7. As the oxidation number of a metal increases, ionic character decreases, for example, Mn2O7 is covalent.
Mostly higher oxides are acidic in nature, Mn2O7 dissolves in water to give permanganic acid (HMnO4), similarly CrO3 gives chromic acid (H2CrO4) and dichromic acid (H2Cr2O7). Generally lower oxides may be amphoteric or basic, for example, Chromium (III) oxide – Cr2O3, is amphoteric and Chromium(II) oxide, CrO, is basic in nature.
Potassium Dichromate K2Cr2O7
Preparation:
Potassium dichromate is prepared from chromate ore. The ore is concentrated by gravity separation. It is then mixed with excess sodium carbonate and lime and roasted in a reverbratory furnace.
![]()
The roasted mass is treated with water to separate soluble sodium chromate from insoluble iron oxide. The yellow solution of sodium chromate is treated with concentrated sulphuric acid which converts sodium chromate into sodium dichromate.
![]()
The above solution is concentrated to remove less soluble sodium sulphate. The resulting solution is filtered and further concentrated. It is cooled to get the crystals of Na2SO4.2H2O.
The saturated solution of sodium dichromate in water is mixed with KCl and then concentrated to get crystals of NaCl. It is filtered while hot and the filtrate is cooled to obtain K2Cr2O7 crystals.
![]()
Physical Properties:
Potassium dichromate is an orange red crystalline solid which melts at 671K and it is moderately soluble in cold water, but very much soluble in hot water. On heating it decomposes and forms Cr2O3 and molecular oxygen. As it emits toxic chromium fumes upon heating, it is mainly replaced by sodium dichromate.
![]()
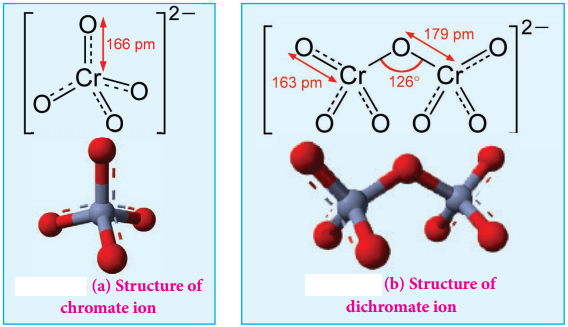
Structure of Dichromate Ion:
Both chromate and dichromate ion are oxo anions of chromium and they are moderately strong oxidizing agents. In these ions chromium is in +6 oxidation state. In an aqueous solution, chromate and dichromate ions can be interconvertible, and in an alkaline solution chromate ion is predominant, whereas dichromate ion becomes predominant in acidic solutions. Structures of these ions are shown in the figure.
Chemical Properties:
1. Oxidation
Potassium dichromate is a powerful oxidising agent in acidic medium. Its oxidising action in the presence of H+ ions is shown below. You can note that the change in the oxidation state of chromium from Cr6+ to Cr3+. Its oxidising action is shown below.
Cr2O72- + 14H+ + 6e– → 2Cr3+ + 7H2O
The oxidising nature of potassium dichromate (dichromate ion) is illustrated in the following examples.
(i) It oxidises ferrous salts to ferric salts.
Cr2O72- + 6Fe2+ + 14H+ → 2Cr3+ + 6Fe3+ + 7H2O
(ii) It oxidises iodide ions to iodine
Cr2O72- + 6I– + 14H+ → 2Cr3+ + 3I2 + 7H2O
(iii) It oxidises sulphide ion to sulphur
Cr2O72- + 3S2- + 14H+ → 2Cr3+ + 3S + 7H2O
(iv) It oxidises sulphur dioxide to sulphate ion
Cr2O72- + 3SO2 + 2H+ → 2Cr3+ + 3SO2-4 + H2O
(v) It oxidises stannous salts to stannic salt
Cr2O72- + 3Sn2+ + 14H+ → 2Cr3+ + 3Sn4+ + 7H2O
(vi) It oxidises alcohols to acids.
2K2Cr2O7 + 8H2SO4 + 3CH3CH2OH →
2K2SO4 + 2Cr2(SO4)3 + 3CH3COOH + 11H2O
Chromyl Chloride Test:
When potassium dichromate is heated with any chloride salt in the presence of Conc H2SO4, orange red vapours of chromyl chloride (CrO2Cl2) is evolved. This reaction is used to confirm the presence of chloride ion in inorganic qualitative analysis.
![]()
The chromyl chloride vapours are dissolved in sodium hydroxide solution and then acidified with acetic acid and treated with lead acetate. A yellow precipitate of lead chromate is obtained.
CrO2Cl2 + 4NaOH → Na2CrO4 + 2NaCl + 2H2O
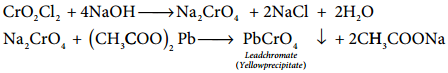
Uses of Potassium Dichromate:
Some important uses of potassium dichromate are listed below.
- It is used as a strong oxidizing agent.
- It is used in dyeing and printing.
- It used in leather tanneries for chrome tanning.
- It is used in quantitative analysis for the estimation of iron compounds and iodides.
Potassium Permanganate – KMnO4
Preparation:
Potassium permanganate is prepared from pyrolusite (MnO2) ore. The preparation involves the following steps.
(i) Conversion of MnO2 to potassium manganate:
Powdered ore is fused with KOH in the presence of air or oxidising agents like KNO3 or KClO3. A green coloured potassium manganate is formed.
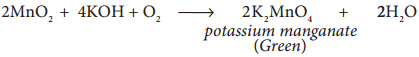
(ii) Oxidation of potassium manganate to potassium permanganate:
Potassium manganate thus obtained can be oxidised in two ways, either by chemical oxidation or electrolytic oxidation.
Chemical Oxidation:
In this method potassium manganate is treated with ozone (O3) or chlorine to get potassium permanganate.
2MnO42- + O3 + H2O → 2MnO4– + 2OH– + O2
2MnO42- + Cl2 → 2MnO4– + 2Cl–
Electrolytic Oxidation
In this method aqueous solution of potassium manganate is electrolyzed in the presence of little alkali.
K2MnO4 ⇄ 2K+ + MnO42-
H2O ⇄ H+ + OH–
Manganate ions are converted into permanganate ions at anode.
![]()
H2 is liberated at the cathode.
2H+ + 2e– → H2↑
The purple coloured solution is concentrated by evaporation and forms crystals of potassium permanganate on cooling.
Physical Properties:
Potassium permanganate exists in the form of dark purple crystals which melts at 513 K. It is sparingly soluble in cold water but, fairly soluble in hot water.
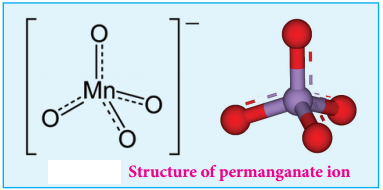
Structure of Permanganate ion
Permanganate ion has tetrahedral geometry in which the central Mn7+ is sp3 hybridised.
Chemical Properties:
1. Action of Heat:
When heated, potassium permanganate decomposes to form potassium manganate and manganese dioxide.
2KMnO4 → 2K2MnO4 + MnO2 + O2
2. Action of conc H2SO4
On treating with cold conc H2SO4, it decomposes to form manganese heptoxide, which subsequently decomposes explosively.
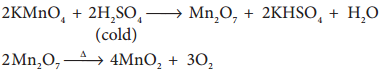
But with hot conc H2SO4, Potassium permanganate give MnSO4.
![]()
3. Oxidising Property:
Potassium permanganate is a strong oxidising agent, its oxidising action differs in different reaction medium.
(a) In neutral medium:
In neutral medium, it is reduced to MnO2
MnO4– + 2H2O + 3e– → MnO2 + 4OH–
(i) It oxidises H2S to sulphur
2MnO4– + 2H2O + 3e– → MnO2 + 4OH–
(ii) It oxidises thiosulphate into sulphate
8MnO4– + 3S2O32- + H2O → 6SO42- + 8MnO2 + 2OH–
(b) In alkaline medium:
In the presence of alkali metal hydroxides, the permanganate ion is converted into manganate.
MnO4– + e– → MnO42-
This manganate is further reduced to MnO2 by some reducing agents.
MnO42- + H2O → MnO2 + 2OH– + [O]
So the overall reaction can be written as follows.
MnO4– + 2H2O + 3e– → MnO2 + 4OH–
This reaction is similar as that for neutral medium.
Bayer’s Reagent:
Cold dilute alkaline KMnO4 is known as Bayer’s reagent. It is used to oxidise alkenes into diols. For example, ethylene can be converted into ethylene glycol and this reaction is used as a test for unsaturation.
(c) In acid medium:
In the presence of dilute sulphuric acid, potassium permanganate acts as a very strong oxidising agent. Permanganate ion is converted into Mn2+ ion.
MnO4– + 8H+ + 5e– → Mn2+ + 4H2O
The oxidising nature of potassium permanganate (permanganate ion) in acid medium is illustrated in the following examples.
(i) It oxidises ferrous salts to ferric salts.
2MnO4– + 10Fe2+ + 16H+ → 2Mn2+ + 10Fe3+ + 8H2O
(ii) It oxidises iodide ions to iodine
2MnO4– + 10 I– + 16H+ → 2Mn2+ + 5I2 + 8H2O
(iii) It oxidises oxalic acid to CO2
2MnO4– + 5(COO)2-2 + 16H+ → 2Mn2+ + 10CO2 + 8H2O
(iv) It oxidises sulphide ion to sulphur
2MnO–4 + 5 S2- + 16H+ → 2Mn2+ + 5 S + 8H2O
(v) It oxidises nitrites to nitrates
2MnO4– + 5 NO2– + 6H+ → 2Mn2+ + 5NO–3 + 3H2O
(vi) It oxidises alcohols to aldehydes.
2KMnO4 + 3H2SO4 + 5CH3CH2OH → K2SO4 + 2MnSO4 + 5CH3CHO + 8H2O
(vii) It oxidises sulphite to sulphate
2MnO4– + 5SO32- + 6H+ → 2Mn2+ + 5SO42- + 3H2O
Uses of Potassium Permanganate:
Some important uses of potassium permanganate are listed below.
- It is used as a strong oxidizing agent.
- It is used for the treatment of various skin infections and fungal infections of the foot.
- It used in water treatment industries to remove iron and hydrogen sulphide from well water.
- It is used as Bayer’s reagent for detecting unsaturation in an organic compound.
- It is used in quantitative analysis for the estimation of ferrous salts, oxalates, hydrogen peroxide and iodides.
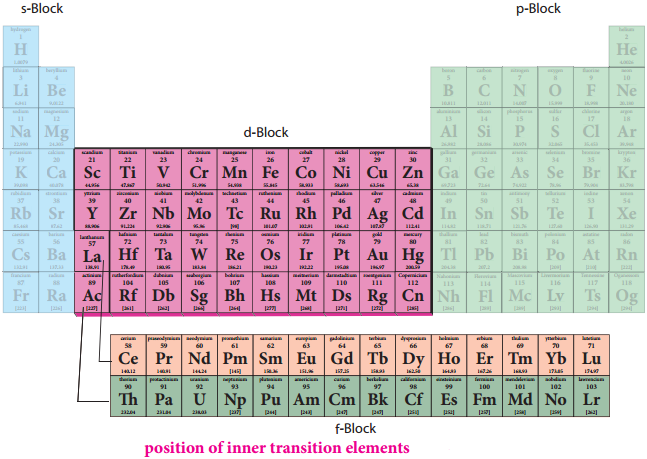
F-Block Elements – Inner Transition Elements
In the inner transition elements there are two series of elements.
- Lanthanoids (previously called lanthanides)
- Actinoids (previously called actinides)
Lanthanoid series consists of fourteen elements from Cerium (58Ce) to Lutetium (71Lu) following Lanthanum (57La). These elements are characterised by the preferential filling of 4f orbitals, Similarly actinoids consists of 14 elements from Thrium (90Th) to Lawrencium (103Lr) following Actinium (89Ac). These elements are characterised by the preferential filling of 5f orbital.
The position of Lanthanoids in the periodic table
The actual position of Lanthanoids in the periodic table is at group number 3 and period number 6. However, in the sixth period after lanthanum, the electrons are preferentially filled in inner 4f sub shell and these fourteen elements following lanthanum show similar chemical properties. Therefore these elements are grouped together and placed at the bottom of the periodic table. This position can be justified as follows.
- Lanthanoids have general electronic configuration [Xe] 4f1-14 5d10-1 6s2
- The common oxidation state of lanthanoides is +3
- All these elements have similar physical and chemical properties.
Similarly the fourteen elements following actinium resemble in their physical and chemical properties. If we place these elements after Lanthanum in the periodic table below 4d series, the properties of the elements belongs to a group would be different and it would affect the proper structure of the periodic table. Hence a separate position is provided to the inner transition elements as shown in the figure.
Electronic Configuration of Lanthanoids:
We know that the electrons are filled in different orbitals in the order of their increasing energy in accordance with Aufbau principle. As per this rule after filling 5s, 5p and 6s and 4f level begin to fill from lanthanum, and hence the expected electronic configuration of Lanthanum(La) is [Xe] 4f1 5d° 6s2 but the actual electronic configuration of Lanthanum is [Xe] 4f° 5d1 6s2 and it belongs to d block.
Filling of 4f orbital starts from Cerium (Ce) and its electronic configuration is [Xe] 4f1 5d1 6s2. As we move from Cerium to other elements the additional electrons are progressively filled in 4f orbitals as shown in the table.
Table: Electronic Configuration of Lanthanum and Lanthanoids
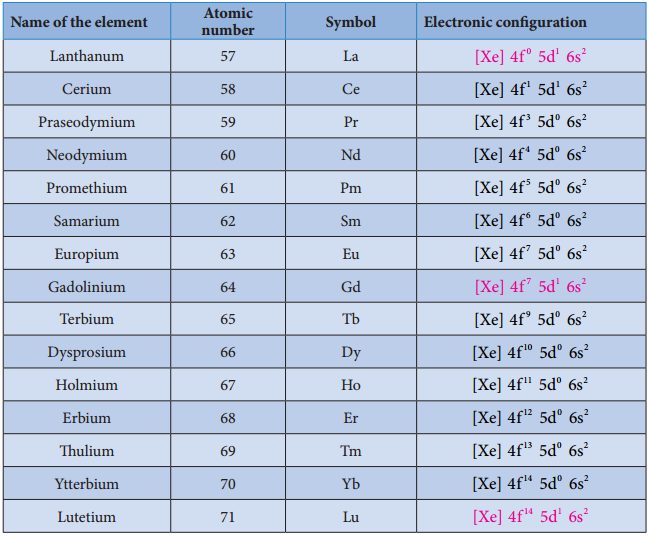
In Gadolinium (Gd) and Lutetium (Lu) the 4f orbitals, are half-filled and completely filled, and one electron enters 5d orbitals. Hence the general electronic configuration of 4f series of elements can be written as [Xe] 4f1-14 5d0-1 6s2
Oxidation State of Lanthanoids:
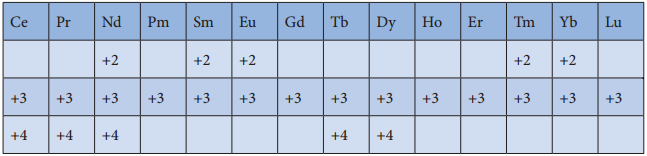
The common oxidation state of lanthanoids is +3. In addition to that some of the lanthanoids also show either +2 or +4 oxidation states. Gd3+ and Lu3+ ions have extra stability, it is due to the fact that they have exactly half filled and completely filled f-orbitals respectively their electronic configurations are
Gd3+: [Xe]4f7
Lu3+: [Xe]4f14
Similarly Cerium and terbium attain 4f° and 4f7 configurations respectively in the +4 oxidation states. Eu2+
and Yb2+ ions have exactly half filled and completely filled f orbitals respectively.
The stability of different oxidation states has an impact on the properties of these elements the following table shows the different oxidation states of lanthanoids.
Atomic and Ionic Radii:
As we move across 4f series, the atomic and ionic radii of lanthanoids show gradual decrease with increse in atomic number. This decrease in ionic size is called lanthanoid contraction.
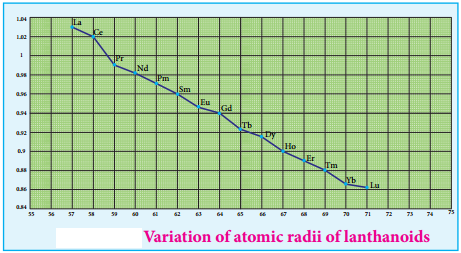
Cause of Lanthanoid Contraction:
As we move from one element to another in 4f series (Ce to Lu) the nuclear charge increases by one unit and an additional electron is added into the same inner 4f sub shell. We know that 4f sub shell have a diffused shapes and therefore the shielding effect of 4f elelctrons relatively poor hence, with increase of nuclear charge, the valence shell is pulled slightly towards nucleus. As a result, the effective nuclear charge experienced by the 4f elelctorns increases and the size of Ln3+ ions decreases. Lanthanoid contraction of various lanthanoids is shown in the graph.
Consequences of Lanthanoid Contraction:
1. Basicity Differences
As we from Ce3+ to Lu3+, the basic character of Ln3+ ions decrease. Due to the decrease in the size of Ln3+ ions, the ionic character of Ln – OH bond decreases (covalent character increases) which results in the decrease in the basicity.
2. Similarities Among Lanthanoids:
In the complete f – series only 10 pm decrease in atomic radii and 20 pm decrease in ionic radii is observed because of this very small change in radii of lanthanoids, their chemical properties are quite similar.
The elements of the second and third transition series resemble each other more closely than the elements of the first and second transition series. For example
|
Series |
Element |
Atomic Radius |
| 3d Series | Ti | 132 pm |
| 4d Series | Zr | 145 pm |
| 5d Series | Hf | 144 pm |
Actinoids:
The fourteen elements following actinium, i.e., from thorium (Th to lawrentium (Lr) are called actinoids. Unlike the lanthanoids, all the actinoids are radioactive and most of them have short half lives. Only thorium and uranium (U) occur in significant amount in nature and a trace amounts of Plutonium (Pu) is also found in Uranium ores. Neptunium (Np) and successive heavier elements are produced synthetically by the artificial transformation of naturally occuring elements by nuclear reactions. Similar to lanthanoids, they are placed at the bottom of the periodic table.
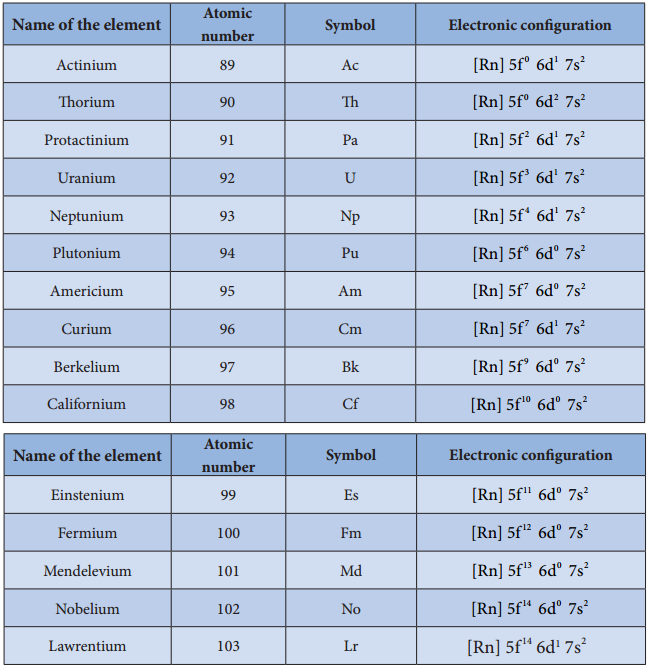
Electronic Configuration:
The electronic configuration of actinoids is not definite. The general valence shell electronic configuration of 5f elements is represented as [Rn]5f1-146d0-27s2. The following table show the electronic configuration of actinoids.
Table: Electronic configuration of actinoids
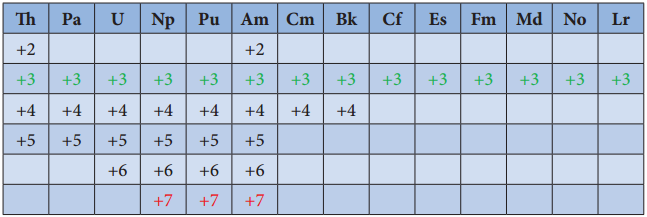
Oxidation State of Actinoids:
Like lanthanoids, the most common state of actinoids is +3. In addition to that actinoids show variable oxidation states such as +2 , +3 , +4 ,+5,+6 and +7. The elements Americium(Am) and Thrium (Th show +2 oxidation state in some compounds, for example thorium iodide (ThI2). The elements T, Pa, U, Np, Pu and Am show +5 oxidation states. Np and Pu exhibit +7 oxidation state.
Differences Between Lanthanoids and Actinoids:
|
Lanthanoids |
Actinoids |
| 1. Differentiating electron enters in 4f orbital | 1. Differentiating electron enters in 5f orbital |
| 2. Binding energy of 4f orbitals are higher | 2. Binding energy of 5f orbitals are lower |
| 3. They show less tendency to form complexes | 3. They show greater tendency to form complexes |
| 4. Most of the lanthanoids are colourless | 4. Most of the actinoids are coloured. For example. U3+(red), U4+(green), UO22+(yellow) |
| 5. They do not form oxo cations | 5. They do form oxo cations such as UO22+, NPO22+ etc |
| 6. Besides +3 oxidation states lanthanoids show +2 and +4 oxidation states in few cases | 6. Besides +3 oxidation states actinoids show higher oxidation states such as +4, +5, +6 and +7 |