In this page, we are providing Is Matter Around Us Pure Class 9 Extra Questions and Answers Science Chapter 2 pdf download. NCERT Extra Questions for Class 9 Science Chapter 2 Is Matter Around Us Pure with Answers will help to score more marks in your CBSE Board Exams. https://ncertmcq.com/extra-questions-for-class-9-science/
Class 9 Science Chapter 2 Extra Questions and Answers Is Matter Around Us Pure
Extra Questions for Class 9 Science Chapter 2 Is Matter Around Us Pure with Answers Solutions
Is Matter Around Us Pure Class 9 Extra Questions Very Short Answer Type
Class 9 Science Chapter 2 Extra Questions And Answers Question 1.
What is the reason for the difference in properties of solutions, colloids and suspensions?
Answer:
Due to different particle size.
Is Matter Around Us Pure Extra Questions Question 2.
Is rain water or distilled water a pure substance?
Answer:
Yes, because it contains particles (molecules) of only water.
Is Matter Around Us Pure Class 9 Extra Questions Question 3.
Can we separate a mixture of alcohol and water by a separating funnel?
Answer:
No, the two liquids are miscible.
Ch 2 Science Class 9 Extra Questions Question 4.
Give two examples of metalloids.
Answer:
Silicon and germanium.
Class 9 Chemistry Chapter 2 Extra Questions Question 5.
Give an example of a solution in which solid is a solute as well as the solvent.
Answer:
Alloys are solid in solid solutions. For example, brass contains about 30% zinc and 70% copper. Here, zinc is the solute while copper is the solvent.
Class 9 Science Ch 2 Extra Questions Question 6.
What type of liquid mixture will kerosesne oil and water form? How will you separate it?
Answer:
Immiscible. We can separate this mixture by using a separating funnel.
Class 9 Is Matter Around Us Pure Extra Questions Question 7.
Define the term heterogeneous.
Answer:
Heterogeneous means that the substance does not have the same properties or characteristics throughout its bulk.
Chapter 2 Science Class 9 Extra Questions Question 8.
Write the constituent element of potassium hydroxide and sodium chloride.
Answer:
The constituent element of potassium hydroxide is K, H and O and sodium chloride is Na and Cl.
Ncert Class 9 Science Chapter 2 Extra Questions Question 9.
On the basis of composition, how is matter classified?
Answer:
- Pure substance
- Mixture.
Extra Questions Of Chapter 2 Science Class 9 Question 10.
What are different categories of pure substances?
Answer:
- Elements
- Compounds.
Class 9 Chapter 2 Science Extra Questions Question 11.
What are the different kinds of mixture?
Answer:
- Homogeneous mixture
- Heterogeneous mixture.
Is Matter Around Us Pure Class 9 Important Questions With Answers Question 12.
What are the constituents of brass?
Answer:
Brass is an alloy and is a mixture of zinc (30%) and copper (70%).
Class 9 Science Chapter 2 Extra Questions Question 13.
How are elements further classified?
Answer:
Metals, non-metals, metalloids.
Extra Questions Of Is Matter Around Us Pure Question 14.
A solution of water and alcohol contains 30 g of water and 60 g of alcohol. What is the concentration of solution?
Answer:
\(\frac { 30 }{ 30 + 60 }\) x 100 = \(\frac { 30 }{ 90 }\) x 100 = 33.3%.
Class 9th Science Chapter 2 Extra Questions Question 15.
What are aqueous solutions?
Answer:
Solutions in which water is the solvent are called aqueous solutions, e.g., sugar solution, in which sugar is dissolved in water.
Question 16.
What is an unsaturated solution?
Answer:
A solution in which some more solute can be dissolved at any fixed temperature is called an unsaturated solution.
Question 17.
How many gram of water is needed to make 8% mass by mass percentage of sodium carbonate solution if 4 g of sodium carbonate is a variable to make a solution?
Answer:
8% means 8 g in 100 g of solution. So if 4 g Na2CO3 is present, it means solution must be 50 g.
Question 18.
What are the conditions required to convert air into liquid air?
Answer:
200 atmospheric pressure and – 200°C.
Question 19.
Define the term inter-conversion of matter.
Answer:
The phenomenon of change of one state of matter into another and back to the original state is called inter-conversion of matter.
Question 20.
Why do fish go in deep water during day light?
Answer:
During day time, the shallow water is warmed and hence it has less dissolved oxygen. Therefore fish tend to go in deep water during day time.
Question 21.
Out of colloid, solution and a suspension which one can be separated by filtration.
Answer:
Suspension.
Question 22.
Out of colloid, solution and a suspension which has the smallest particle?
Answer:
Solution.
Is Matter Around Us Pure Class 9 Extra Questions Short Answer Type 1
Question 1.
Suggest separation technique(s) one would need to employ to separate the following mixtures. [NCERT Exemplar]
(a) Mercury and water
(b) Potassium chloride and ammonium chloride
(c) Common salt, water apd sand
(d) Kerosene oil, water and salt.
Answer:
(a) Separation by using separating funnel
(b) Sublimation
(c) Filtration followed by evaporation
Or
Centrifugation followed by evaporation/distillation
(d) Separation by using separating funnel to separate kerosene oil followed by distillation.
Question 2.
A salt can be recovered from its solution by evaporation. Suggest some other technique for the same? [NCERT Exemplar]
Answer:
Crystallisation.
Question 3.
The ‘sea water’ can be classified as a homogeneous as well as heterogeneous mixture. Comment.
Answer:
- Homogeneous – mixture of salts and water only.
- Heterogeneous – contains salts, water, mud, decayed plant, etc.
Question 4.
Fill in the following blanks:
(i) Milk is a ……………… solution but vinegar is a solution.
(ii) Milk is a colloidal solution in which ……………… is the dispersed phase and ……………… is the dispersion medium.
Answer:
(i) colloidal, true
(ii) fat, water.
Question 5.
(a) Classify Brass and Diamond as element and mixture.
(b) How is a chemical change different from a physical change?
Answer:
(a) Brass is homogeneous mixture also called alloy. The constituents are Cu and Zn. Diamond is an element. It is an allotropic form of carbon.
(b) In a chemical change, a new substance is formed as a result of chemical reaction. No new substance is formed in a physical change.
Question 6.
Identify the dispersed phase and dispersion medium in the following examples of colloids:
(a) Fog
(b) Cheese
(c) Coloured gemstone.
Answer:
(a) Fog: Liquid (water drops) acts as dispersed phase and gas (air) as the dispersion medium.
(b) Cheese: Solid (fat) acts as the dispersed phase and water (liquid) as the dispersion medium.
(c) Coloured gemstone: Solids act the dispersed phase as well as the dispersion medium.
Question 7.
Explain, why particles of a colloidal solution do not settle down when left undisturbed, while in the case of a suspension they do. [NCERT Exemplar]
Answer:
Particle size in a suspension is larger than those in a colloidal solution. Also molecular interaction in a suspension is not strong enough to keep the particles suspended and hence they settle down.
Question 8.
Smoke and fog both are aerosols. In what way are they different? [NCERT Exemplar]
Answer:
Both fog and smoke have gas as the dispersion medium. The only difference is that the dispersed phase in fog is liquid and in smoke it is a solid.
Question 9.
How will you bring about the following separation?
(i) Fine mud particles floating in water.
(ii) Carbon particles present in smoke.
Answer:
(i) By coagulation using alum and then filtering.
(ii) By passing smoke through electric plates maintained at a high potential difference. The colloidal particles of carbon get neutralised and fall down while air escapes out.
Question 10.
Classify the following as Physical or chemical properties. [NCERT Exemplar]
(a) The composition of a sample of steel is 98% iron, 1.5% carbon and 0.5% other elements.
(b) Zinc dissolves in hydrochloric acid with the evolution of hydrogen gas.
(c) Metallic sodium is soft enough to be cut with a knife.
(d) Most metal oxides form alkalies on interacting with water.
Answer:
Physical properties: (a) and (c)
Chemical properties: (b) and (d)
Question 11.
Suggest a suitable separation technique for the following: [NCERT Exemplar]
(a) Mercury and water
(b) Coloured components from blue ink
(c) Ammonium chloride and potassium chloride
(d) Mixture of alcohol and water.
Answer:
(a) The separation can be done by the use of a separating funnel. Mercury forms the lower layer (heavier) and water the upper layer (lighter).
(b) The separation can be done with the help of chromatography.
(c) Process of sublimation can be used. Ammonium chloride collects as the sublimate while potassium chloride remains in the dish.
(d) Process of fractional distillation can be used. Alcohol (ethyl alcohol) with lower boiling point (78°C or 351 K) gets distilled leaving behind water with higher boiling point (100°C or 373 K) in the distillation flask.
Question 12.
Name the type of colloids in each of the following giving an example of each.
| Dispersed Phase | Dispersing Medium | |
| A | Liquid | Gas |
| B | Liquid | Liquid |
| C | Liquid | Solids |
Answer:
| A | Aerosol | (Fog) |
| B | Emulsion | (Milk) |
| C | Gel | (Jelly) |
Question 13.
You are given two samples of water labelled as ‘A’ and ‘B’ Sample ‘A’ boils at 100°C and sample ‘B’ boils at 102°C. Which sample of water will not freeze at 0°C? Comment. [NCERT Exemplar]
Answer:
Sample ‘B’ will not freeze at 0°C because it is not pure water. At 1 atm, the boiling point of pure water is 100°C and the freezing point of pure water is 0°C.
Question 14.
Identify colloids from the following: Copper sulphate solution, milk, smoke, muddy water, butter, sugar solution, face cream, lemonade.
Answer:
Colloids: milk, smoke, muddy water, butter, face cream, lemonade.
Question 15.
What are the favourable qualities given to gold when it is alloyed with copper or silver for the purpose of making ornaments? [NCERT Exemplar]
Answer:
Pure gold is very soft as compared to gold alloyed with silver or copper. Thus for providing strength to gold, it is alloyed.
Question 16.
12 mL of dettol is added to a beaker containing 500 mL of water and stirred. State four observations that you make.
Answer:
When dettol is added to a beaker containing of water, the following observations are made.
- An emulsion is formed which is of colloidal nature.
- The colour of emulsion is milky.
- It gives characteristic smell of dettol.
- The solution can pass through a filter paper.
Is Matter Around Us Pure Class 9 Extra Questions Short Answer Type 2
Question 1.
During an experiment the students were asked to prepare a 10% (Mass/Mass) solution of sugar in water. Ramesh dissolved 10 g of sugar in 100 g of water while Sarika prepared it by dissolving 10 g of sugar in water to make 100 g of the solution. [NCERT Exemplar]
(a) Are the two solutions of the same concentration?
(b) Compare the mass % of the two solutions.
Answer:
(a) No.
\(Mass\%=\frac{\text { Mass of solute }}{\text { Mass of solute }+\text { Mass of solvent }} \times 100\)
(b) Solution made by Ramesh
Mass % = \(\frac { 10 }{ 10 + 100 }\) = \(\frac { 10 }{ 110 }\) x 100 = 9.09%
solution made by sarika
Mass % = \(\frac { 10 }{ 100 }\) x 100 = 10%
The solution prepared by Sarixa has a higher mass % than that prepared by Ramesh.
Question 2.
State which of the following solutions exhibit Tyndall effect:
Starch solution, Sodium chloride solution, Tincture of iodine, Air.
Answer:
(i) Tyndall effect is shown both by starch solution and air which are heterogeneous mixtures and have the capacity to scatter a beam of light as it passes through them.
(ii) Sodium chloride solution and tincture of iodine (iodine crystals dissolved in ethyl alcohol) are both homogeneous in nature and do not exhibit any Tyndall effect.
Question 3.
While diluting a solution of salt in water, a student by mistake added acetone (boiling point 56°C). What technique can be employed to get back the acetone? Justify your choice. [NCERT Exemplar]
Answer:
Distillation, since acetone is more volatile it will separate out first.
Question 4.
(a) Why is crystallisation technique better than evaporation?
(b) Write any two physical properties of each of metals and non-metals.
(c) Name the technique used to separate butter from curd.
Answer:
(a) Both these techniques are used to separate solid substances from their solutions. But crystallisation is considered better because during evaporation certain solids may decompose or some of them like sugar get charred when the solution is evaporated completely to dryness. As a result of crystallisation, even the shapes of the crystals do not change.
(b) (i) Metals have a shiny surface known as lustre.
(ii) Metals are malleable and ductile.
(iii) Non-metals are mostly poor conductors of electricity.
(iv) Non-metals are generally soft.
(c) Butter can be separated from curd by the process of centrifugation. This is usually done by churning which is very common as well as convenient.
Question 5.
Name the process associated with the following:
(a) Dry ice is kept at room temperature and at one atmospheric pressure.
(b) A drop of ink placed on the surface of water contained in a glass spreads throughout the water.
(c) A potassium permanganate crystal is in a beaker and water is poured into the beaker with stirring.
(d) A acetone bottle is left open and the bottle becomes empty.
(e) Milk is churned to separate cream from it.
(f) Settling of sand when a mixture of a sand and water is left undisturbed for some time.
Answer:
(a) Sublimation
(b) Diffusion
(c) Dissolution/diffusion
(d) Evaporation, diffusion
(e) Centrifugation
(f) Sedimentation
Question 6.
On dissolving chalk powder in water, a suspension is obtained. Give any four reasons to support the fact that the mixture so obtained is a suspension only.
Answer:
It is supported by the following reasons:
- White particles of chalk powder can be seen with the naked eyes.
- The particles can be separated by ordinary filter paper.
- Upon shaking, a white turbidity reappears in solution.
- Light cannot pass through the suspension which shows that it is of opaque nature.
Question 7.
Give an example for each of the following:
(a) Solid-liquid homogeneous mixture
(b) Gas-gas homogeneous mixture
(c) Liquid-liquid heterogeneous mixture.
Answer:
(a) Mixture of sodium chloride in water.
(b) Air. It is a homogeneous mixture of a number of gases.
(c) Emulsion of oil and water.
Question 8.
What would you observe when
(a) a saturated solution of potassium chloride prepared at 60°C is allowed to cool to room temperature?
(b) an aqueous sugar solution is heated to dryness?
(c) a mixture of iron filings and sulphur powder is heated strongly?
Answer:
(a) Solid potassium chloride will separate out.
(b) Initially the water will evaporate and then sugar will get charred.
(c) Iron sulphide will be formed.
Question 9.
(a) Arrange solids, liquids and gases in increasing order of the following properties of matter
(i) rigidity
(ii) diffusion
(iii) compressibility.
(b) Write one example from your daily life which is based on diffusion of gases.
Answer:
(a) (i) Rigidity: Gases < Liquids < Solids
(ii) Diffusion: Solids < Liquids < Gases
(iii) Compressibility: Solids < Liquids < Gases.
(b) Smell of aroma or perfume released in one corner of the room soon spreads in the whole room.
Question 10.
Is air a mixture or a compound? Give three reasons.
Answer:
Air is a mixture and not a compound as discussed below:
(i) The properties of a mixture are in between those of its constituents. The two major components of air are oxygen (20.9% by volume) and nitrogen (78.1% by volume). In oxygen, any fuel bums brightly but in nitrogen it gets extinguished. In contrast, in air the fuel bums slowly.
(ii) The components of a mixture can be separated by simple physical methods. For example, the components of air can be separated by fractional distillation of liquid air.
(iii) The composition of a mixture is variable. The composition of air is also variable. It has more oxygen in the country side than in big cities.
(iv) When air is obtained by mixing its constituent gases, no heat is either evolved or absorbed.
(v) Liquid air does not have a fixed boiling point.
Is Matter Around Us Pure Class 9 Extra Questions Long Answer Type
Question 1.
Classify each of the following, as a physical or a chemical change. Give reasons.
(a) Drying of a shirt in the Sun.
(b) Rising of hot air over a radiator.
(c) Burning of kerosene in a lantern.
(d) Change in the colour of black tea on adding lemon juice to it.
(e) Churning of milk cream to get butter.
Answer:
Physical changes: (a), (b), (e)
Chemical changes: (c), (d)
Question 2.
Iron filings and sulphur were mixed together and divided into two parts, ‘A’ and ‘B’. Part ‘A’ was heated strongly while Part ‘B’ was not heated. Dilute hydrochloric acid was added to both the parts and evolution of gas was seen in both the cases. How will you identify the gases evolved?
Answer:
Part A
Part B
![]()
When dilute HCl is added to it, only the iron filings in the mixture react and sulphur remains unreacted
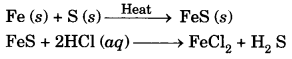
Fe (s) + 2 HCl (aq) → FeCl2 + H2 gas
H2S gas formed has a foul smell and on passing through lead acetate solution, it turns the solution black. Hydrogen gas burns with a pop sound.
Question 3.
Fill in the blanks:
(a) A colloid is a ……………….. mixture and its components can be separated by the technique known as ………………..
(b) Ice, water and water vapour look different and display different ……………….. properties but they are the same.
(c) A mixture of chloroform and water taken in a separating funnel is mixed and left undisturbed for sometime. The upper layer in the separating funnel will be of ……………….. and the lower layer will be that of
(d) A mixture of two or more miscible liquids, for which the difference in the boiling points is less than 25 K can be separated by the process called ………………..
(e) When light is passed through water containing a few drops of milk, it shows a bluish tinge. This is due to the ……………….. of light by milk and the phenomenon is called ……………….. This indicates that milk is a ……………….. solution. [NCERT Exemplar]
Answer:
(a) heterogeneous, centrifugation
(b) physical, chemically
(c) water, chloroform (hint-density of water is less than that of chloroform)
(d) fractional distillation scattering, Tyndall effect, colloidal.
Question 4.
Give an example each for the mixture having the following characteristics. Suggest a suitable method to separate the components of these mixtures. [NCERT Exemplar]
(a) A volatile and a non-volatile component.
(b) Two volatile components with appreciable difference in boiling points.
(c) Two immiscible liquids.
(d) One of the components changes directly from solid to gaseous state.
(e) Two or more coloured constituents soluble in some solvent.
Answer:
(a) Evaporation or distillation
(b) Distillation
(c) By using a separating funnel
(d) Sublimation
(e) Chromatography
Question 5.
Which separation techniques you will apply for the separation of the following mixtures?
(a) Oil from water
(b) Camphor from sand
(c) Sodium chloride from its solution in water
(d) Cream from milk
(e) Metal pieces from engine oil of a car.
Answer:
(a) By the use of a separating funnel.
(b) With the help of sublimation technique
(c) By evaporation crystallisation technique.
(d) By the use of a centrifuge
(e) By the use of filtration technique
Is Matter Around Us Pure Class 9 Extra Questions HOTS
Question 1.
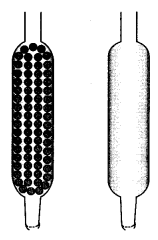
Which of the tubes shown below will be more effective as a condenser in the distillation apparatus? [NCERT Exemplar]
Answer:
The presence of beads in tube (a) would provide a larger surface area for cooling. So, this tube will be more effective as a condenser.
Question 2.
Non-metals are usually poor conductors of heat and electricity. They are non-lustrous, non- sonorous, non-malleable and are coloured.
(a) Name a lustrous non-metal.
(b) Name a non-metal which exists as a liquid at room temperature.
(c) The allotropic form of a non-metal is a good conductor of electricity. Name the allotrope.
(d) Name a non-metal which is known to form the largest number of compounds.
(e) Name a non-metal other than carbon which shows allotropy.
(f) Name a non-metal which is required for combustion.
Answer:
(a) Iodine
(b) Bromine
(c) Graphite
(d) Carbon
(e) Sulphur, phosphorus
(f) Oxygen.
Question 3.
The teacher instructed three students ‘A’, ‘B’ and ‘C’ respectively to prepare a 50% (mass by volume) solution of sodium hydroxide (NaOH). ‘A’ dissolved 50 g of NaOH in 100 mL of water, ‘B’ dissolved 50 g of NaOH in 100 g of water while ‘C’ dissolved 50 g of NaOH in water to make 100 mL of solution. Which one of them has made the desired solution and why?
Answer:
‘C’ has made the desired solution.
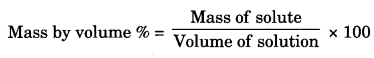
= \(\frac { 50 }{ 100 }\) x 100
= 50% mass by volume.
Question 4.
On heating calcium carbonate gets converted into calcium oxide and carbon dioxide.
(a) Is this a physical or a chemical change?
(b) Can you prepare one acidic and one basic solution by using the products formed in the above process? If so, write the chemical equation involved.
Answer:
(a) Chemical change
(b) Acidic and basic solutions can be prepared by dissolving the products of the above process in water
CaO + H2O → Ca(OH)2 (basic solution)
CO2 + H2O → H2CO3 (acidic solution)
Question 5.
Arun has prepared 0.01% (by mass) solution of sodium chloride in water. Which of the following correctly represents the composition of the solutions?
(a) 1.00 g of NaCl + 100 g water
(b) 0.11 g of NaCl + 100 g of water
(c) 0.01 g of NaCl + 99.99 g of water
(d) 0.10 g of NaCl + 99.90 g of water
Answer:
(c) 0.01 g of NaCl + 99.99 g of water
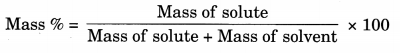
= \(\frac { 0.01 }{ 0.01 + 99.99 }\) x 100
= \(\frac { 0.01 }{ 100 }\) x 100
= 0.01 g
Question 6.
A child wanted to separate the mixture of dyes constituting a sample of ink. He marked a line by the ink on the filter paper and placed the filter paper in a glass containing water as shown in the figure. The filter paper was removed when the water moved near the top of the filter paper. [NCERT Exemplar]
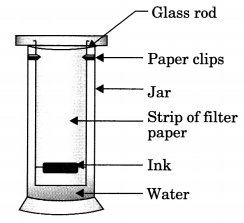
- What would you expect to see, if the ink contains three different coloured components?
- Name the technique used by the child.
- Suggest one more application of this technique.
Answer:
- Three different bands will be observed.
- Chromatography
- To separate the pigments present in chlorophyll.
Question 7.
A group of students took an old shoe box and covered it with a black paper from all sides. They fixed a source of light (a torch) at one end of the box by making a hole in it and made another hole on the other side to view the light. They placed a milk sample contained in a beaker/tumbler in the box as shown in the figure. They were amazed to see that milk taken in the tumbler was illuminated. They tried the same activity by taking a salt solution but found that light simply passed through it?
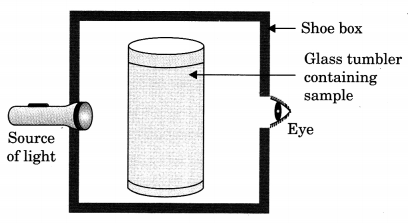
- Explain why the milk sample was illuminated. Name the phenomenon involved.
- Same results were not observed with a salt solution. Explain.
- Can you suggest two more solutions which would show the solution?
Answer:
- Milk is a colloid and would show Tyndall effect.
- Salt solution is a true solution and would not scatter light.
- Detergent solution, sulphur solution.
Question 8.
Sudha tested the solubility of four salts X, Y, Z and T at different temperatures and collected the following data. (Solubility refers to the amount in grams dissolved in 100 g of water to give a saturated solution.)
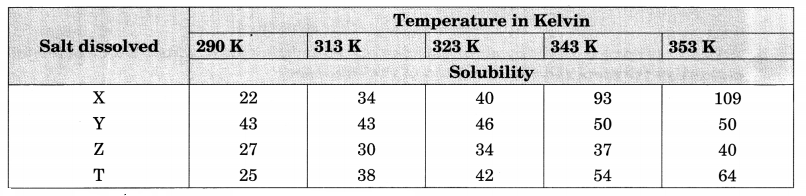
Answer the following questions from the table:
- Which salt has the highest and lowest solubility at 323 K?
- A student prepared a saturated solution of X at 323 K and then added 25 g water to it. What mass of X must be added to again make the solution saturated?
- The solubility of which salt is least affected by increase in temperature?
- What mass of T would be required to make saturated solution in 200 g of water at 290 K?
Answer:
1. At 323 K, salt Y has the highest solubility in water while salt Z has the lowest solubility.
2. By definition of saturated solution,
100 g of water at 323 K contain salt = 40 g
125 g of water at 323 K contain salt = \(\frac { 40 g }{ 100 g }\) x (125 g) = 50 g
∴ Mass of salt to be added to make the solution again saturated = (50 – 40) = 10 g
3. The data show that the solubility of the salt Y is least affected with increase in temperature.
4. At 290 K, mass of T required to make a saturated solution in 200 g of water = \(\frac { 25 g }{ 100 g }\) x (200 g) = 50 g
Is Matter Around Us Pure Class 9 Extra Questions Value Based (VBQs)
Question 1.
Mallika’s mother was suffering from cold and cough. Mallika prepared tea for her mother. She boiled water in a pan, then she added tea leaves, sugar and milk to it. She filtered the tea in a cup and served it to her mother.
(a) Explain the values shown by Mallika.
(b) Identify solute, solvent, residue and filtrate in this activity.
Answer:
(a) Mallika used the knowledge of chemistry to provide relief to her mother. Actually she prepared an extract of tea leaves which is helpful in curing cold and cough and gives warmth to the body.
(b) Solute: Tea leaves and sugar.
Solvent: water and milk.
Filtrate: homogeneous mixture of water, milk, sugar and extract of tea leaves.
Question 2.
Amit was asked by his teacher to separate a liquid mixture of acetone and ethyl alcohol. He set up a distillation apparatus and tried to distil the mixture. To his surprise, both the liquids got distilled. Teacher told Amit to repeat the experiment by using a fractionating column in the distillation flask. Amit followed the advice of the teacher and he was able to separate the two liquids.
- Why was Amit not successful in separating the liquid mixture earlier?
- Why did teacher ask him to use the fractionating column?
- Which liquid was distilled first?
- As a student of chemistry, what value based information you have gathered?
Answer:
1. The difference in boiling point temperatures of acetone (56°C) and ethyl alcohol (78°C) is only 22°C.
Therefore, process of simple distillation fails in this case.
2. Fractionating column is quite effective in this case because it obstructs the distillation of ethyl alcohol which is high boiling and at the same time helps in the distillation of acetone which is low boiling.
3. Acetone was distilled first since it has comparatively low boiling point.
4. The process of simple distillation can be used only in case, the liquids present in the mixture differ in their boiling point by 25°C or more.
