Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Iupac Nomenclature
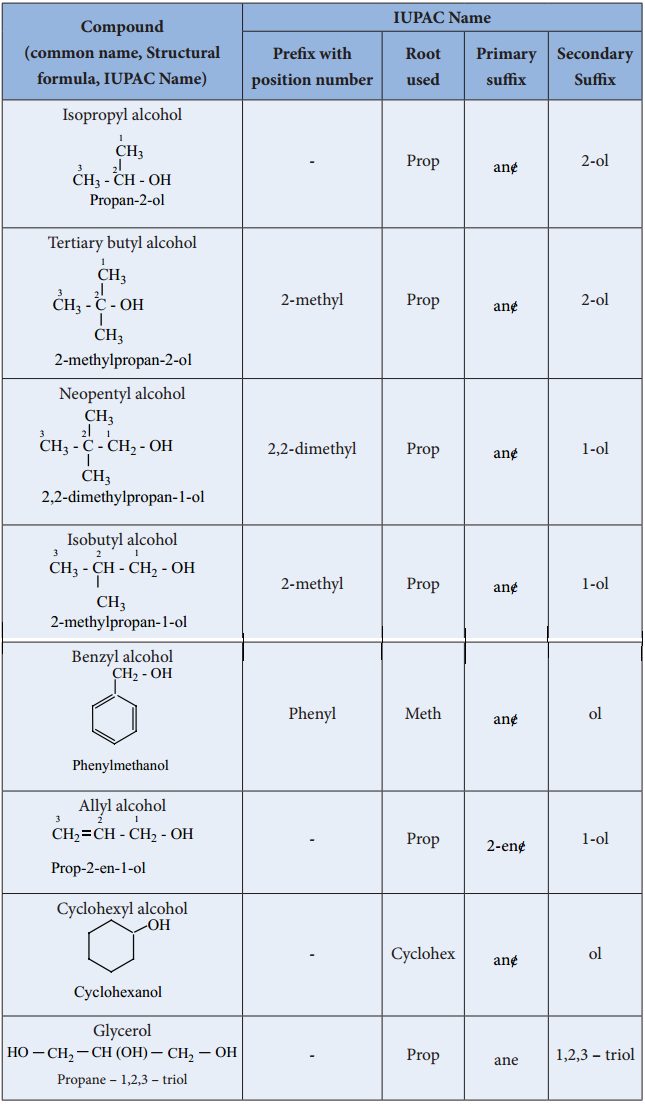
We have already learnt about naming the organic compounds according to IUPAC guidelines in XI standard. Let us recall the basic rules to name the alcohols.
- Select the longest continuous chain of carbon atoms (root word) containing the functional group (- OH).
- Number the carbon atoms in the chain so that the carbon bearing the – OH group has the lowest possible number.
- Name the substituent (if any)
- Write the name of the alcohol as below.
![]()
The following table illustrates the IUPAC nomenclature of alcohols.
Structure of the Functional Group of Alcohol
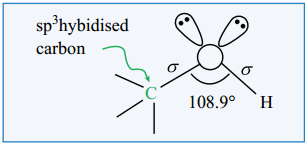
The structure of -O-H group which is attached to a sp3 hybridised carbon is similar to the structure of -O-H group attached to a hydrogen in water. i.e., ‘V’ shaped.
In such alcohols, one of the sp3 hybridised orbital of oxygen linearly overlap with the sp3 hybridised orbital of carbon to form a C-O, ‘σ’ bond and another sp3 hybridised orbital linearly overlap with 1s orbital of hydrogen to form a O-H ‘σ’ bond. The remaining two sp3 hybridised orbitals of oxygen are occupied by two lone pairs of electrons. Due to the lone pair – lone pair repulsion, the C-O-H bond angle in methanol is reduced to 108.9° from the regular tetrahedral bond angle of 109.5°.
Preparation of Alcohols:
We have already learnt that the nucleophilic substitution reactions of alkyl halides with dilute alkali, conversion of alkenes to alcohols by hydration and the preparation of alcohols using Grignard reagent in XI standard. These reactions are summarised below.
1. From Alkyl Halides:
Alkyl halides on heating with dilute aqueous NaOH gives alcohols. Primary alkyl halides undergo substitution by SN2 reaction. Secondary and tertiary alkyl halides usually undergo nucleophilic substitution by SN1 mechanism.
![]()
If R = t-butyl, the reaction proceeds through the formation of t-butyl carbocation
2. From Alkenes:
Addition of water across the double bond of an alkene in presence of concentrated sulphuric acid gives alcohols. This addition reaction follows Markownikof ’s rule.
Example:
![]()
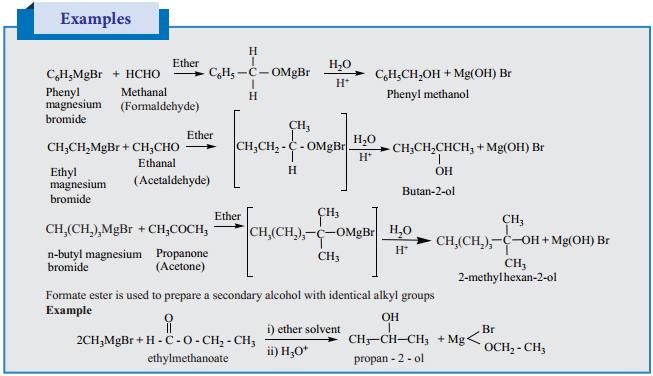
3. From Grignard Reagent:
Nucleophilic addition of Grignard reagent to aldehydes/ketones in presence of dry ether followed by the acid hydrolysis gives alcohols. Formaldehyde gives primary alcohol and other aldehydes give secondary alcohols. Ketones give tertiary alcohols.
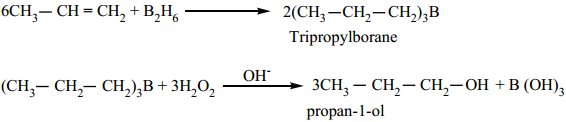
4. Hydroboration:
Diborane reacts with an alkene to form trialkyl borane which on treatment with H2O2 in presence of NaOH
gives an alcohol. (Refer reactions of diborane) The overall reaction is hydration of an alkene. This reaction yields an anti-Markownikof ‘s product.
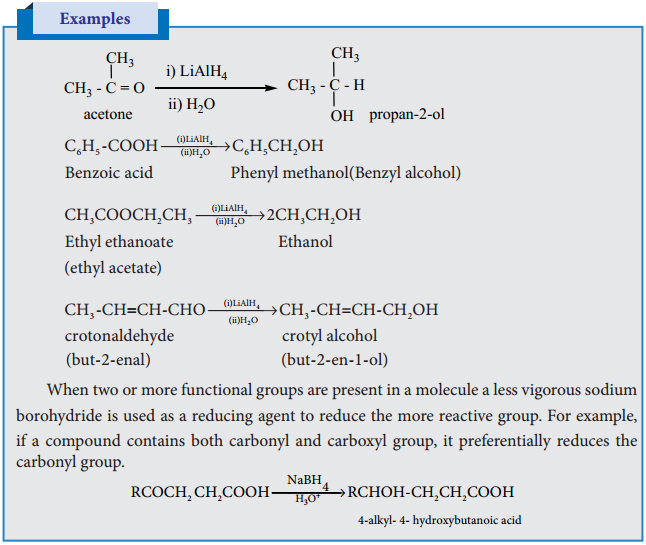
5. Reduction of Carbonyl Compounds:
Reduction of aldehydes/ketones with LiAlH4 in the presence of solvents like THF (Tetrahydrofuran) followed by hydrolysis gives alcohols. Unlike other reducing agents such as Raney Ni, Na-Hg/H2O, the lithium aluminium hydride does not reduce the carbon-carbon double bond present in unsaturated carbonyl compound and hence it is a best reagent to prepare unsaturated alcohols.
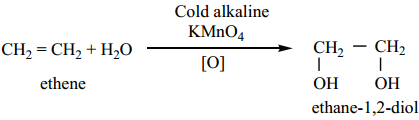
Preparation of Glycol
We have already learnt that the hydroxylation of ethylene using cold alkaline solution of potassium permanganate (Baeyer’s reagent) gives ethylene glycol.
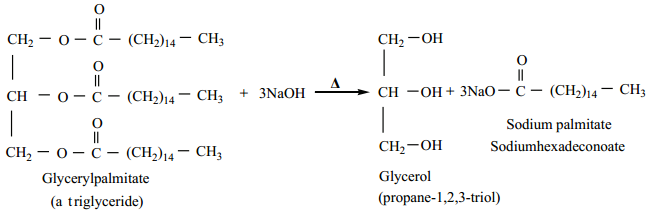
Preparation of Glycerol
Glycerol occurs in many natural fats and it is also found in long chain fatty acids in the form of glyceryl esters (Triglycerides). The alkaline hydrolysis of these fats gives glycerol and the reaction is known as saponification.
Methods to Differentiate Primary, Secondary and Tertiary Alcohols.
The following tests are used to distinguish between 1°, 2° and 3° alcohols.
(a) Lucas Test:
When alcohols are treated with Lucas agent (a mixture of concentrated HCl and anhydrous ZnCl2) at room temperature, tertiary alcohols react immediately to form a turbidity due to the formation of alkyl chloride which is insoluble in the medium. Secondary alcohols react within 10 minutes to form a turbidity of alkyl chloride where primary alcohols do not react at room temperature.
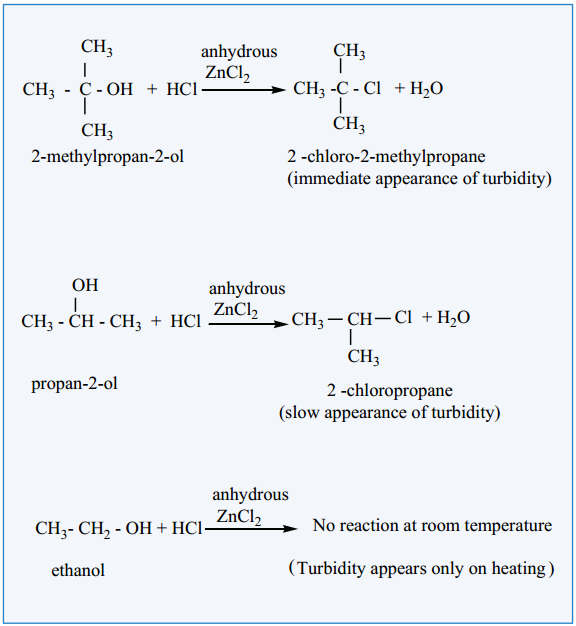
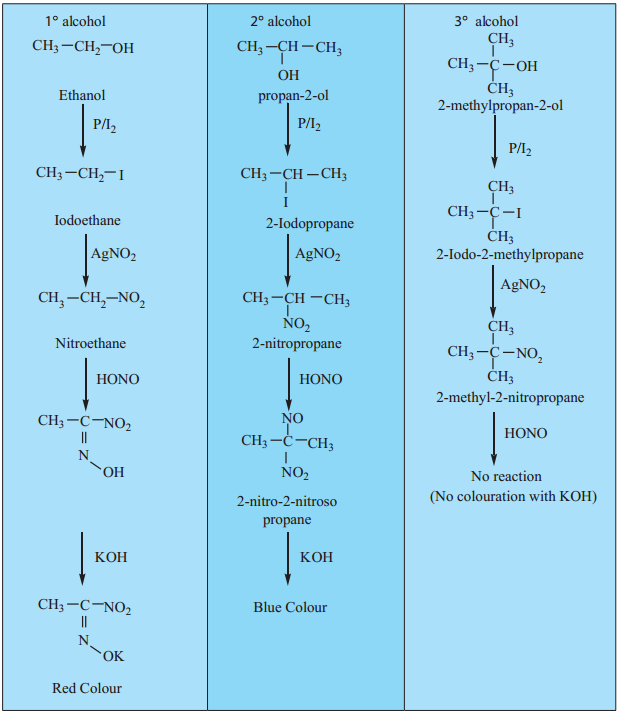
(b) Victor Meyer’s Test:
This test is based on the behaviour of the different nitro alkanes formed by the three types of alcohols with nitrous acid and it consists of the following steps.
- Alcohols are converted into alkyl iodide by treating it with I2/P.
- Alkyl iodide so formed is then treated with AgNO2 to form nitro alkanes.
- Nitro alkanes are fially treated with HNO2 (mixture of NaNO2/HCl) and the resultant solution is made alkaline with KOH.
Result:
- Primary alcohol gives red colour
- Secondary alcohol gives blue colour.
- No colouration will be observed in case of tertiary alcohol.
Properties of Alcohols
Physical Properties
- Lower alcohols are colourless liquids and the higher members are waxy solids.
- They have higher boiling points than the corresponding other organic compounds such as alkanes, aldehydes, ethers etc., this is due to the presence of intermolecular hydrogen bonding present in alcohols.
- Among isomeric alcohols primary alcohols have higher boiling point and the tertiary alcohols have lower boiling points.
- The lower members are highly soluble in water due to the formation of intermolecular hydrogen bonding with water.
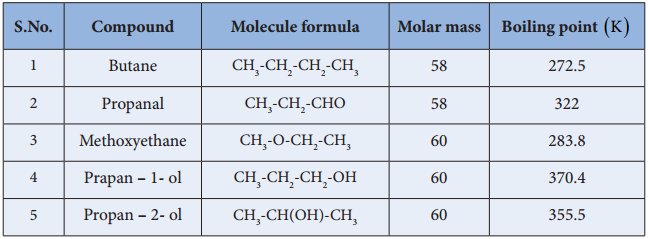
Table: Boiling point of alcohols in comparision with other organic compounds.
Chemical Properties of Alcohols
Nucleophilic Substitution Reactions of Alcohols
Alcohol has a strong basic leaving group (OH–). So, – OH group is first converted into – O+H2 group by adding an acid. The – O+H2 group in the protonated alcohol can be easily displaced by a nucleophile such as Br– to give alkyl halides.
Example:
Alcohols undergo nucleophilic substitution reaction with hydro halic acids to form alkyl halides. In case of tertiary alcohols heating is required.
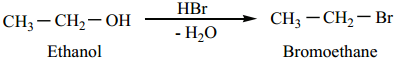
Alkyl Halide Formation from Primary Alcohols Follow SN2 mechanism
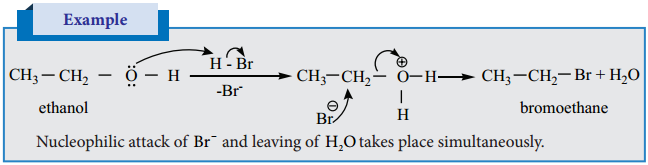
Alkyl Halide Formation of Tertiary Alcohols Follow SN1 mechanism.
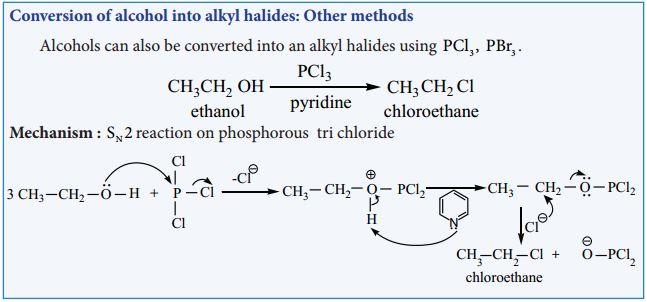
The conversion of an alcohol to alkyl halide can also be effected using thionyl chloride
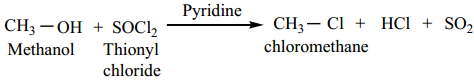
This reaction also follows the SNi mechanism in the presence of pyridine.
Elimination Reactions of Alcohols
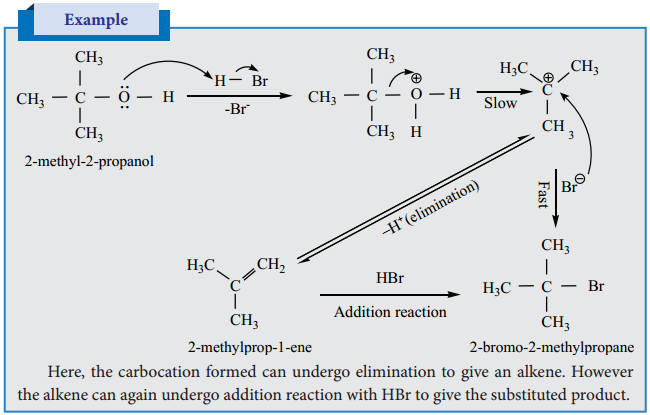
When alcohols are heated with a suitable dehydrating agents like sulphuric acid, the H and OH present in the adjacent carbons of alcohols are lost, and it results in the formation of a carbon – carbon double bond. Phosphoric acid, anhydrous ZnCl2, alumina etc., can also be used as dehydrating agents.
Example:
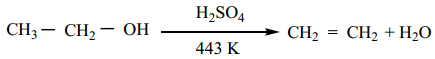
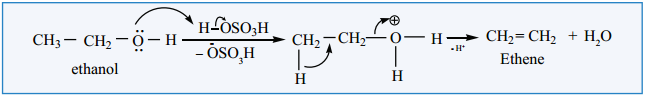
Mechanism
Primary alcohols undergo dehydration by E2 mechanism
Tertiary alcohols undergo dehydration by E1 mechanism. It involves the formation of a carbocation.
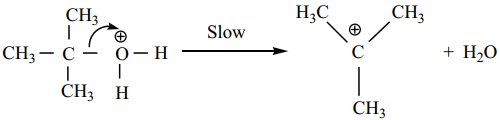
Protonation of Alcohol
Step 1:
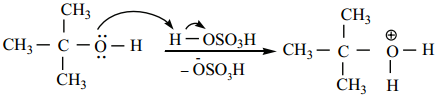
Step 2:
Dissociation of oxonium ion to form a carbonation.
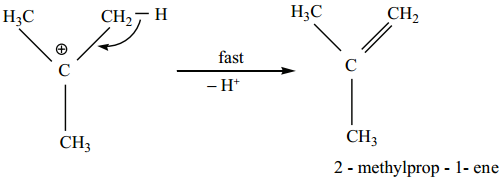
Step 3:
Deprotonation of carbocation to form an alkene
Order of Reactivity:
The relative reactivities of alcohols in the dehydration reaction follows the order
primary < secondary < tertiary

Saytzef ‘s Rule
During intramolecular dehydration, if there is a possibility to form a carbon – carbon double bond at different locations, the preferred location is the one that gives the more (highly) substituted alkene i.e., the stable alkene.
For example, the dehydration of 3, 3 – dimethyl – 2 – butanol gives a mixture of alkenes. The secondary carbocation formed in this reaction undergoes rearrangement to form a more stable tertiary carbocation.
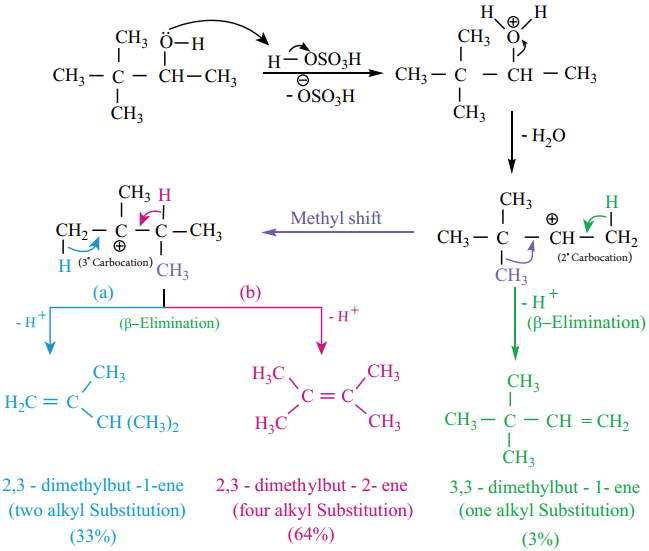
Oxidation of Alcohols
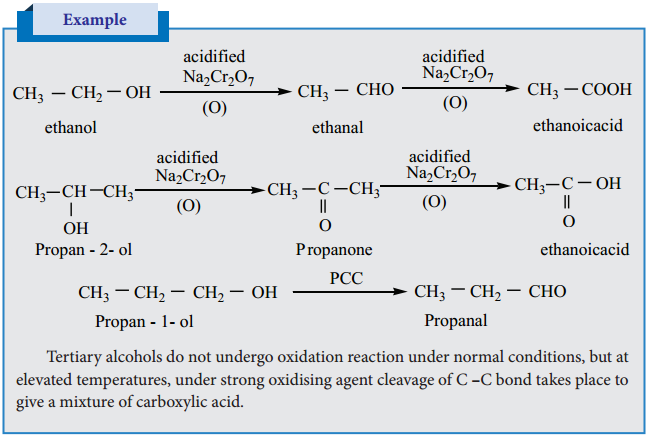
The important reactions of alcohols are their oxidation to give carbonyl compounds. The commonly used oxidising agent is acidified sodiumdichromate. Oxidation of primary alcohols give an aldehyde which on further oxidation gives the carboxylic acids. To stop the oxidation reaction at the aldehyde / ketone stage, pyridinium chlorochromate (PCC) is used as an oxidising agent.
Swern Oxidation
In this method, dimethyl sulfoxide (DMSO) is used as the oxidising agent, which converts alcohols to ketones / aldehydes. In this method an alcohol is treated with DMSO and oxalyl chloride followed by the addition of triethylamine.
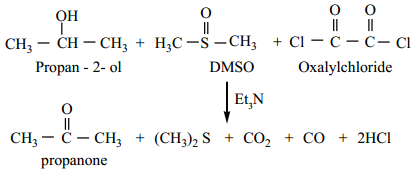
Biological Oxidation
The fermentation of the food consumed by an animal produces alcohol. To detoxify the alcohol, the liver produces an enzyme called alcohol dehydrogenase (ADH). Nicotinamide adenine dinucleotide (NAD) present in the animals act as an oxidising agent and ADH catalyses the oxidation of toxic alcohols into non-toxic aldehyde.
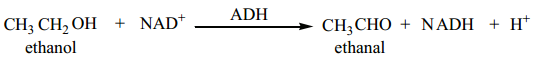
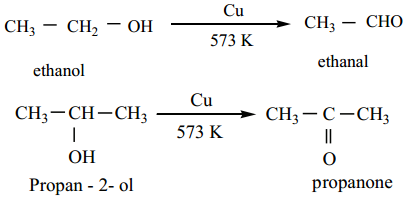
Catalytic Dehydrogenation
When the vapours of a primary or a secondary alcohol are passed over heated copper at 573K, dehydrogenation takes place to form aldehyde or ketone.
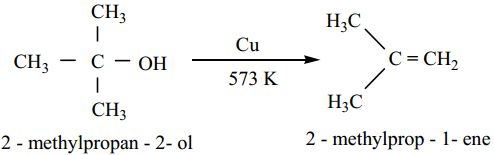
Tertiary alcohols undergo dehydration reaction to give alkenes.
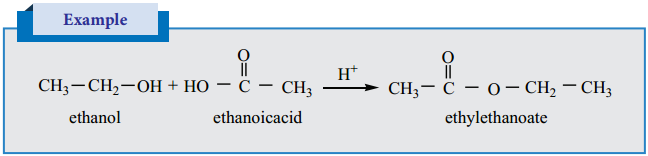
Esterification
Alcohols react with carboxylic acids in the presence of an acid to give esters
Reactions of Glycol
Ethylene glycol contains two primary alcoholic groups and it exhibits the usual reactions of hydroxyl group. Like other primary alcohols, it reacts with metallic sodium to form monosodium glycolate and disodium glycolate. The hydroxyl groups can be converted to the halide groups by treating glycol with halic acid (or with PCl5 / PCl3 / SOCl2)
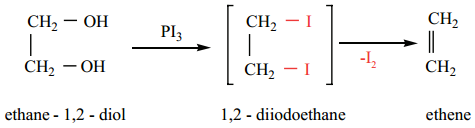
When ethylene glycol is treated with HI or P/I2, 1, 2 – diiodoethane is first formed which decomposes to give ethene.
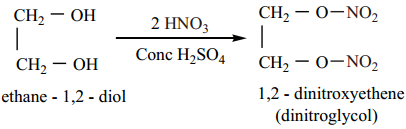
On heating with conc HNO3 in the presence of Con. H2SO4, ethylene glycol forms dinitroglycol.
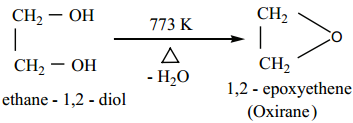
Dehydration Reaction
Ethyleneglycol undergoes dehydration reaction under different conditions to form different products.
1. When heated to 773K, it forms epoxides.
2. When heated with dilute sulphuric acid (or) anhydrous ZnCl2 under pressure in a sealed tube, it gives acetaldehyde.
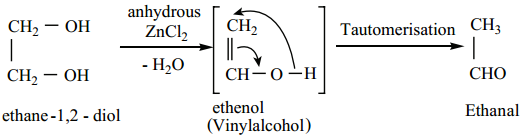
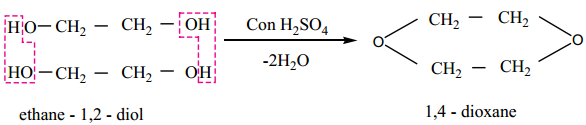
3. When distilled with Conc. H2SO4, glycol forms dioxane
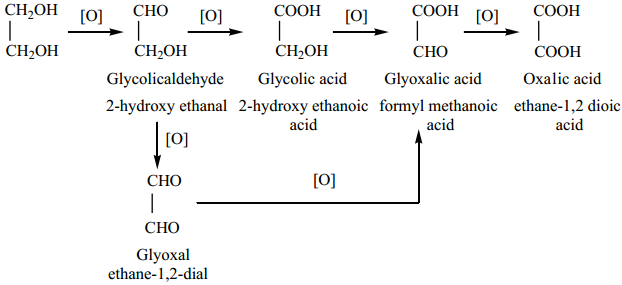
Oxidation of Glycol
On oxidation, glycol gives a variety of products depending on the nature of oxidizing agent and other reaction conditions.
(i) When nitric acid (or) alkaline potassium permanganate is used as the oxidizing agent, the following products are obtained.
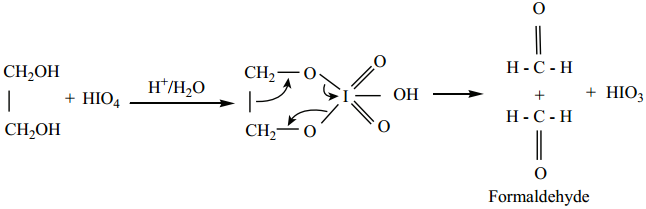
(ii) Oxidation of Glycol with Periodic Acid
Ethylene glycol on treatment with periodic acid gives formaldehyde. This reaction is selective for vicinal 1, 2 – diols and it proceeds through a cyclic periodate ester intermediate.
Reaction of Glycerol
Nitration:
Glycerol reacts with concentrated nitric acid in the presence of concentrated sulphuric acid to form TNG (nitroglycerine).
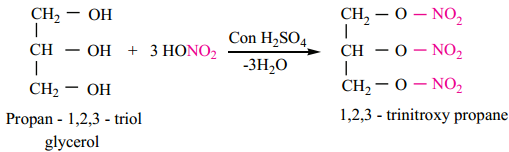
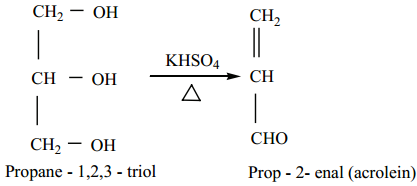
Dehydration
When glycerol is heated with dehydrating agents such as Con H2SO4, KHSO4 etc…., it undergoes dehydration to form acrolein.
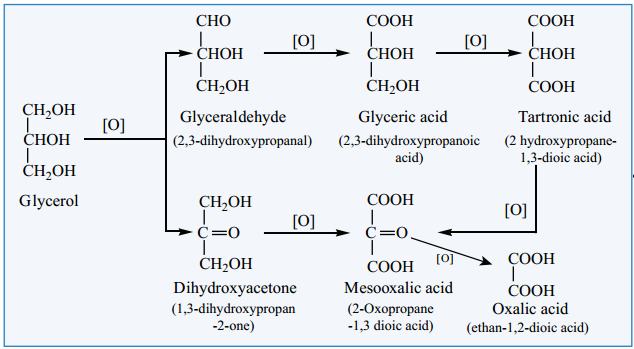
Oxidation
Glycerol can give rise to a variety of oxidation products depending on the nature of the oxidising agent used for oxidation.
- Oxidation of glycerol with dil. HNO3 gives glyceric acid and tartronic acid.
- Oxidation of glycerol with Conc. HNO3 gives mainly glyceric acid.
- Oxidation of glycerol with bismuth nitrate gives as meso oxalic acid.
- Oxidation of glycerol with Br2/H2O (or) NaOBr (or) Fenton’s reagent (FeSO4 + H2O2) gives a mixture of glyceraldehyde and dihydroxy acetone(Ths mixture is named as glycerose).
- On oxidation with HIO4 or Lead tetra acetate (LTA) it gives formaldehyde and formic acid.
- Acidified KMnO4 oxidises glycerol into oxalicacid.
Uses of Alcohols
Uses of Methanol:
- Methanol is used as a solvent for paints, varnishes, shellac, gums, cement, etc.
- In the manufacture of dyes, drugs, perfumes and formaldehyde.
Uses of Ethanol:
1. It is also used in the preparation of
- Paints and varnishes.
- Organic compounds like ether, chloroform, iodoform, etc.,
- Dyes, transparent soaps.
2. As a substitute for petrol under the name power alcohol used as fuel for aeroplane
3. It is used as a preservative for biological specimens.
Uses of Ethylene Glycol:
- Ethylene glycol is used as an antifreeze in automobile radiator
- Its dinitrate is used as an explosive with TNG.
Uses of Glycerol
- Glycerol is used as a sweetening agent in confectionary and beverages.
- It is used in the manufacture of cosmetics and transparent soaps.
- It is used in making printing inks and stamp pad ink and lubricant for watches and clocks.
- It is used in the manufacture of explosive like dynamite and cordite by mixing it with china clay.
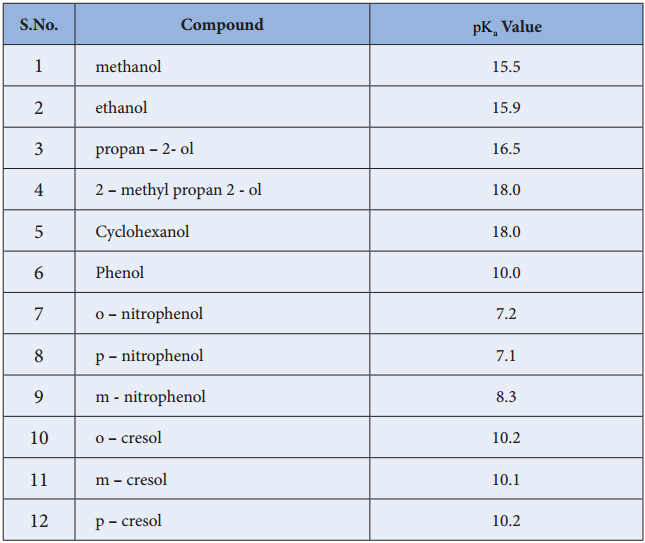
Acidity of Alcohols
According to Bronsted theory, an acid is defined as a proton donor and the acid strength is the tendency to give up a proton. Alcohols are weakly acidic and their acidity is comparable with water. Except methanol, all other alcohols are weaker acid than water. The Ka value for water is 1.8 × 10-16 where as for alcohols, the Ka value in the order 10-18 to 10-16.
Alcohols react with active metals such as sodium, aluminium etc… to form the corresponding alkoxides with the liberation of hydrogen gas and similar reaction to give alkoxide is not observed in the reaction of alcohol with NaOH.
2C2H5 – OH + 2Na → 2C2H5ONa + H2↑
The above reaction explains the acidic nature of alcohols.
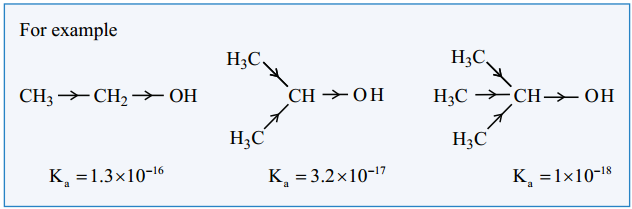
Comparison of acidity of 1°, 2° and 3° alcohols
The acidic nature of the alcohol is due to the polar nature of O – H bond. When an electron withdrawing – I groups such as – Cl, – F etc… is attached to the carbon bearing the OH group, it withdraws the electron density towards itself and thereby facilitating the proton donation.
In contrast, the electron releasing group such as alkyl group increases the electron density on oxygen and decreases the polar nature of O – H bond, Hence it results in the decrease in acidity. On moving from primary to secondary and tertiary alcohols, the number of alkyl groups which attached to the carbon bearing -OH group increases, which results in the following order of acidity.
1° alcohol > 2° alcohol > 3° alcohol
Alcohols can also act as a Bronsted bases. It is due to the presence of unshared electron pairs on oxygen which make them proton acceptors.
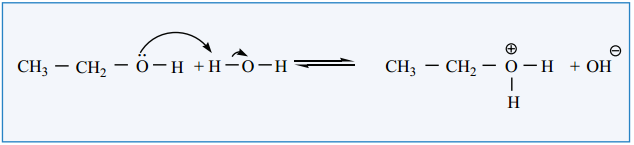
Acidity of Phenol
Phenol is more acidic than aliphatic alcohols. Unlike alcohols it reacts with bases like sodium hydroxide to form sodium phenoxide. This explains the acidic behaviour of phenol let us consider the aqueous solution of phenol in which the following equilibrium exists.
C6H5 – OH + H.OH ⇄ C6H5 – 0Θ + H3O⊕
Ka value for the above equilibrium is 1 × 10-10 at 25°C. This Ka value indicates that it is more acidic than aliphatic alcohols. This increased acidic behaviour can be explained on the basis of the stability of phenoxide ion. We have already learnt in XI standard that the phenoxide is more stabilised by resonance than phenol.
In substituted phenols, the electron withdrawing groups such as – NO2, – Cl enhances the acidic nature of phenol especially when they are present at ortho and para positions. In such cases, there is a possibility for the extended delocalisation of negative charge on the phenoxide ion. On the other hand the alkyl substituted phenols show a decreased acidity due to the electron releasing +I effect of alkyl group.
Table: pKa Values of some Alcohols and Phenols
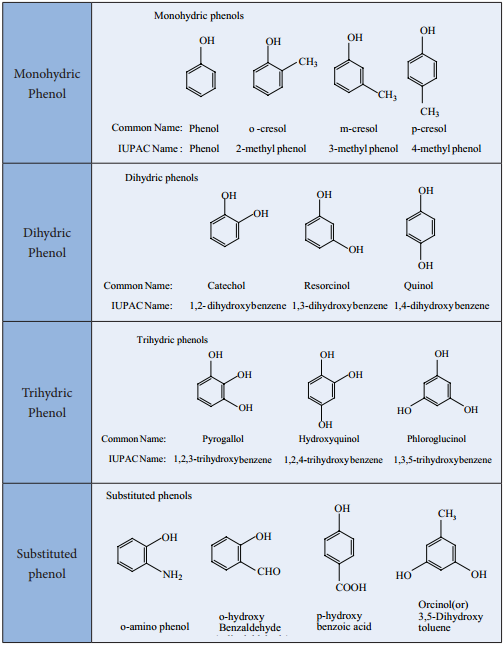
Phenols:
Phenols are organic compounds in which a – OH group is directly attached to a benzene ring. The carbon bearing the – OH group is sp2 hybridized.
Table: Classification of Phenols
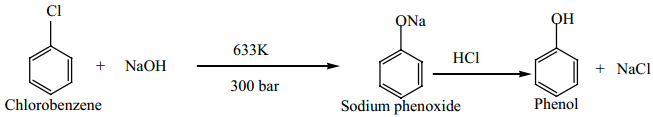
Preparation of Phenols
(a) From Halo Arenes (Dows Process)
When Chlorobenzene is hydrolysed with 6-8% N a O H at 300 bar and 633K in a closed vessel, sodium phenoxide is formed which on treatment with dilute HCl gives phenol.
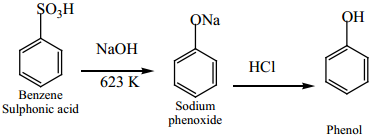
(b) From Benzene Sulphonic Acid
Benzene is sulphonated with oleum and the benzene sulphonic acid so formed is heated with molten NaOH at 623K gives sodium phenoxide which on acidification gives phenol.
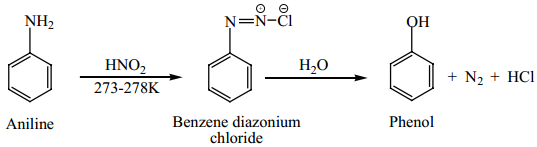
(c) From Aniline
Aniline is diazotized with nitrous acid (NaNO2 + HCl ) at 273-278K to give benzene diazonium chloride which on further treatment with hot water in the presence of mineral acid gives phenol.
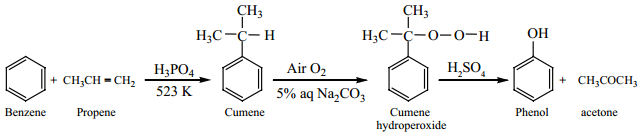
(d) From Cumene
A mixture of benzene and propene is heated at 523K in a closed vessel in presence of H3PO4 catalyst gives
cumene (isopropylbenzene). On passing air to a mixture of cumene and 5% aqueous sodium carbonate solution, cumene hydro peroxide is formed by oxidation. It is treated with dilute acid to get phenol and acetone. Acetone is also an important byproduct in this reaction.
Physical Properties
Phenol is colourless, needle shaped crystal, hygroscopic, corrosive and poisonous. It turns pink on exposure to air and light. The simplest phenols are liquids or low melting solids, they have quite high boiling points. Phenol is slightly soluble in water because of hydrogen bonding. However other substituted phenols are essentially insoluble in water.
Chemical Properties:
Reactions involving – OH group.
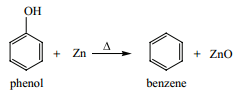
(a) Reaction with Zn Dust:
Phenol is converted to benzene on heating with zinc dust. In this reaction the hydroxyl group which is attached to the aromatic ring is eliminated.
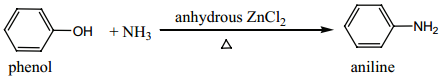
(b) Reaction with Ammonia:
Phenol on heating with ammonia in presence of anhydrous ZnCl2 gives aniline.
(c) Formation of Esters:
Schotten-Baumann Reaction:
Phenol on treatment with acid chlorides gives esters. The acetylation and benzoylation of phenol are called Schotten-Baumann reaction.
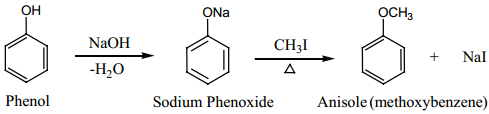
(d) Formation of Ethers:
Williamson Ether Synthesis:
An alkaline solution of phenol reacts with alkyl halide to form phenyl ethers. The alkyl halide undergoes nucleophilic substitution by the phenoxide ion in the presence of alkali.
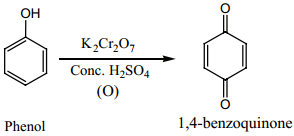
(e) Oxidation:
Phenol undergoes oxidation with air or acidified K2Cr2O7 with conc. H2SO4 to
form 1, 4 – benzoquinone.
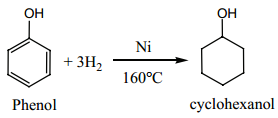
(f) Reduction:
Phenol on catalytic hydrogenation gives cyclohexanol.
Reactions of Benzene Ring:
Electrophilic Aromatic Substitution:
We have already learnt in XI standard that the groups like etc., which when directly attached to the benzene ring, activate the ring towards electrophilic substitution reaction and direct the incoming electrophile to occupy either the ortho or para position.
Common electrophilic aromatic substitutions are as follows:
(i) Nitrosation:
Phenol can be readily nitrosoated at low temperature with HNO2.
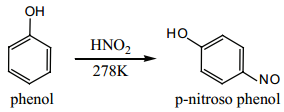
(ii) Nitration:
Phenol can be nitrated using 20% nitric acid even at room temperature, a mixture of ortho and para nitro phenols are formed.
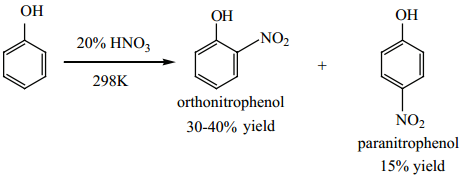
The ortho and para isomers are separated by steam distillation, as o-nitro phenol is slightly soluble in water and more volatile due to intra molecular hydrogen bonding, whereas p-nitro phenol is more soluble in water and less volatile due to intermolecular hydrogen bonding.
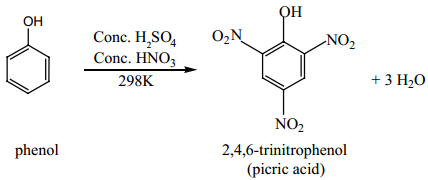
Nitration with Conc. HNO3 + con.H2SO4 gives picric acid.
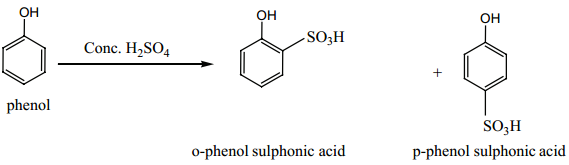
(iii) Sulphonation:
Phenol reacts with con. H2SO4 at 280K to form o-phenol sulphonic acid as the major product. When the reaction is carried out at 373K the major product is p-phenol sulphonic acid.
(iv) Halogenation:
Phenol reacts with bromine water to give a white precipitate of 2, 4, 6-tri bromo phenol.
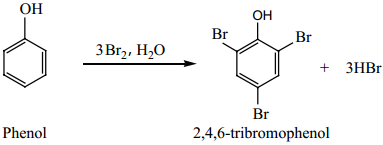
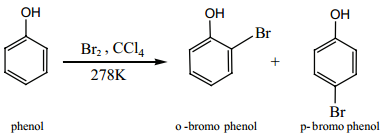
If the reaction is carried out in CS2 or CCl4 at 278K, a mixture of ortho and para bromo phenols are formed.
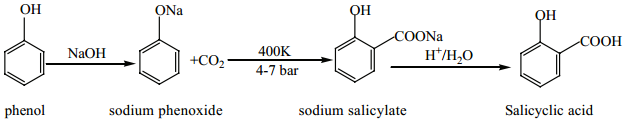
(v) Kolbe’s (or) Kolbe’s Schmit Reaction:
In this reaction, phenol is first converted into sodium phenoxide which is more reactive than phenol towards electrophilic substitution reaction with CO2. Treatment of sodium phenoxide with CO2 at 400K, 4-7 bar pressure followed by acid hydrolysis gives salicylic acid.
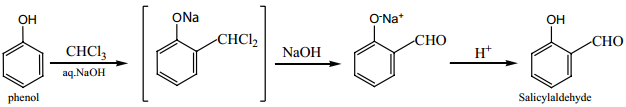
(vi) Riemer – Tiemann Reaction:
On treating phenol with CHCl3/NaOH, a -CHO group is introduced at ortho position. This reaction proceeds through the formation of substituted benzal chloride intermediate.
(vii) Phthalein Reaction:
On heating phenol with phthalic anhydride in presence of con. H2SO4, phenolphthalein is obtained.
(viii) Coupling Reaction:
Phenol couples with benzene diazonium chloride in an alkaline solution to form p-hydroxy azobenzene(a red orange dye).
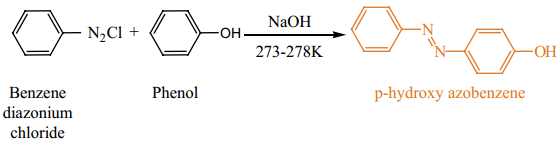
Test to Differentiate Alcohol and Phenols
- Phenol react with benzene diazonium chloride to form a red orange dye, but ethanol has no reaction with it.
- Phenol gives purple colouration with neutral ferric chloride solution, alcohols do not give such coloration with FeCl3.
- Phenol reacts with NaOH to give sodium phenoxide. Ethyl alcohol does not react with NaOH.
Uses of Phenol
- About half of world production of phenol is used for making phenol formaldehyde resin. (Bakelite).
- Phenol is a starting material for the preparation of
- Drugs such as phenacetin, Salol, aspirin, etc.
- Phenolphthalein indicator.
- Explosive like picric acid.
It is used as an antiseptic-carbolic lotion and carbolic soaps.
Ethers:
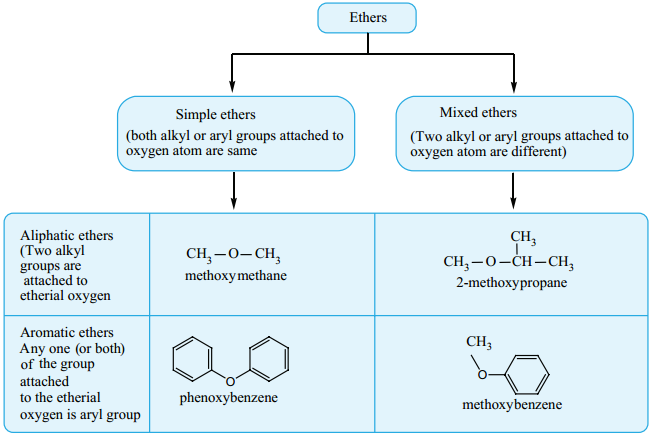
Ethers are a class of organic compound in which an oxygen atom is connected to two alkyl/aryl groups image 66. Ethers can be considered as the derivatives of hydrocarbon in which one hydrogen atom is replaced by an alkoxy (- OR) or an aryloxy (- OAr) group. The general formula of aliphatic ether is CnH2n+2O.
Classification:
Structure of Functional Group
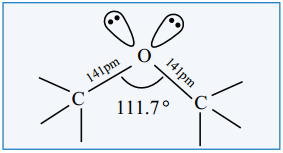
The structure of ethereal oxygen which is attached to two alkyl groups is similar to the structure of -O-H group of alcohol. The oxygen atom is sp3 hybridized. Two sp3 hybridized orbitals of oxygen linearly overlap with two sp3 hybrid orbitals of the carbon which are directly attached to the oxygen forming two C – O ‘σ’ bonds. The C-O-C bond angle is slightly greater than the tetrahedral bond angle due to the repulsive interaction between the two bulkier alkyl groups.
IUPAC System:
Let us recall the naming of ethers according to IUPAC nomenclature.
Preparation of Ethers:
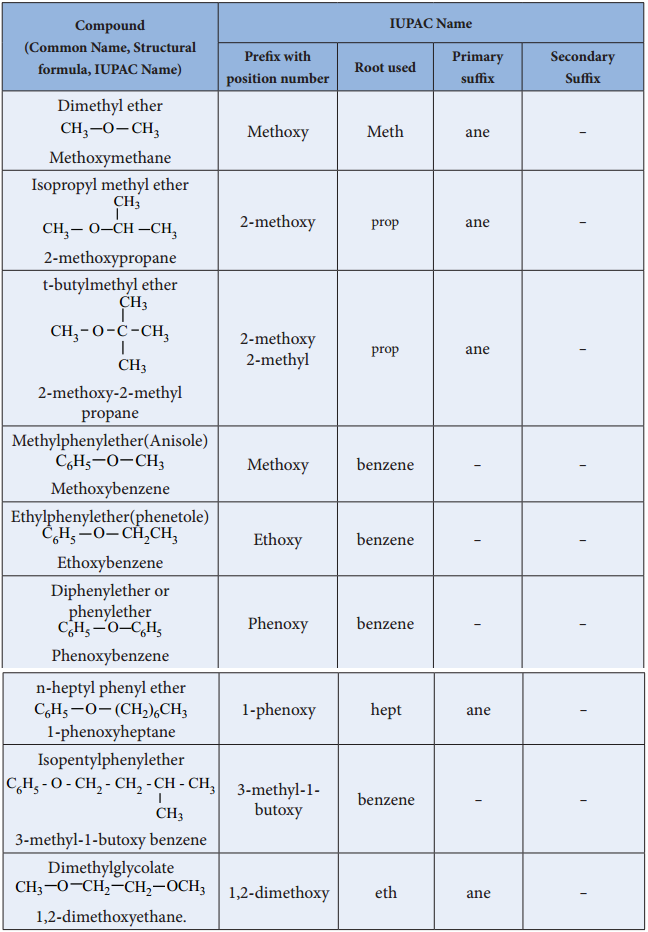
1. Inter Molecular Dehydration of Alcohol.
We have already learnt that when ethanol is treated with con. H2SO4 at 443K, elimination takes place to form ethene. If the same reaction is carried out at 413K, substitution competes over elimination to form ethers.
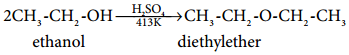
Mechanism:
This method is useful for the preparation of simple ethers and not suitable for preparing mixed ethers. If a mixture of two different alcohols is used, mixture of different ethers will be formed and they are difficult to separate.
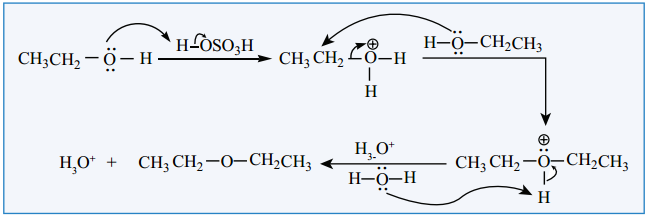
2. Williamsons Synthesis:
When an alkyl halide is heated with an alcoholic solution of sodium alkoxide, the corresponding ethers are obtained. The reaction involves SN2 mechanism.
Mechanism:
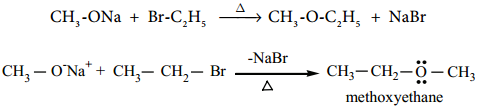
We know that primary alkyl halides are more susceptible for SN2 reaction. Hence for the preparation of mixed ether having primary and tertiary alkyl group, primary alkyl halide and tertiary alkoxide are used. On the other hand, if we use tertiary alkyl halide and primary alkoxide, elimination dominates and succeeds over substitution to form an an alkene.
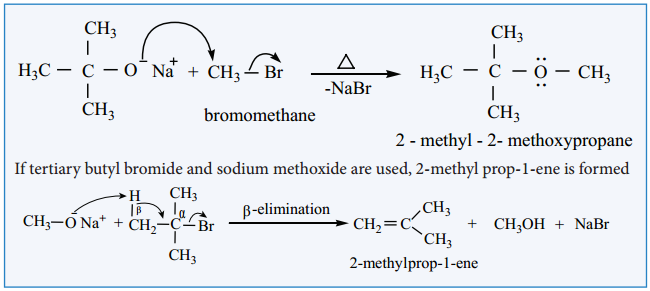
Methylation of Alcohol
Methyl ethers can be prepared by treating an alcohol with diazomethane in presence of catalyst, floroboric acid.
![]()
Physical Properties:
Ethers are polar in nature. The dipolemoment of ether is the vector sum of two polar C-O bonds with significant contribution from two lone pairs of electrons. For example, the dipole moment of diethyl ether is 1.18D. Boiling point of ethers are slightly higher than that of alkanes and lower than that of alcohols of comparable masses.
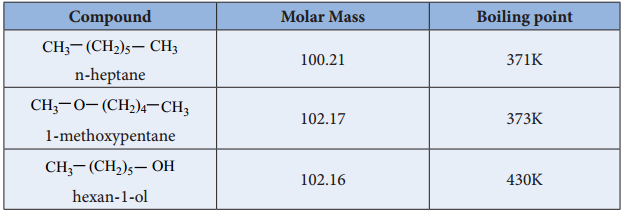
Oxygen of ether can also form Hydrogen bond with water and hence they are miscible with water. Ethers dissolve wide range of polar and non-polar substances.
Chemical Properties of Ethers:
1. Nucleophilic Substitution Reactions of Ethers.
Ethers can undergo nucleophilic substitution reactions with HBr or HI . HI is more reactive than HBr.
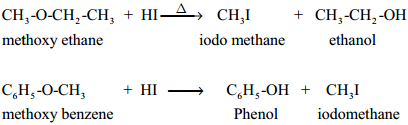
Mechanism:
Ethers having primary alkyl group undergo SN2 reaction while tertiary alkyl ether undergo SN1 reaction.
Protonation of ether is followed by the attack of halide ion. The halide ion preferentially attacks the less sterically hindered of the two alkyl groups which are attached to etherial oxygen.
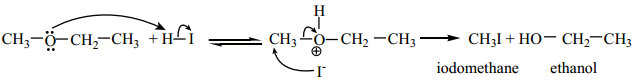
When excess HBr or HI is used, the alcohol formed will further react with HBr or HI to form alkyl halides.
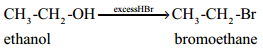
Autooxidation of Ethers:
When ethers are stored in the presence of atmospheric oxygen, they slowly oxidise to form hydroperoxides and dialkylperoxides. These are explosive in nature. Such a spontaneous oxidation by atmospheric oxygen is called autooxidation.
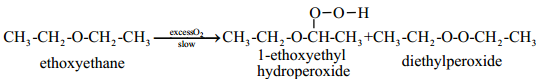
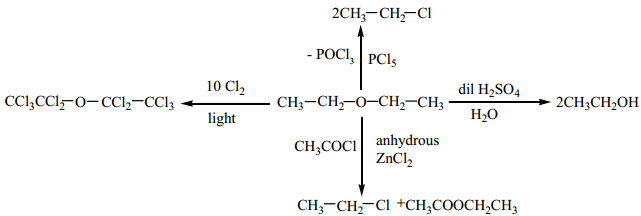
Some of the Reaction of Diethyl Ether.
Aromatic Electrophilic Substitution Reactions:
The alkoxy group (- OR) is an ortho, para directing group as well as activating group. It activates the aromatic ring towards electrophilic substitution.
(i) Halogenation:
Anisole undergoes bromination with bromine in acetic acid even in the absence of a catalyst, para isomer is obtained as the major product.
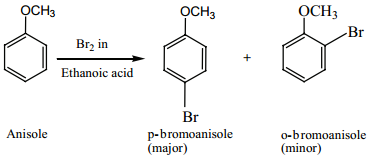
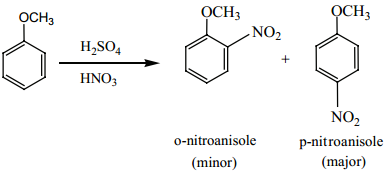
(ii) Nitration:
Anisole reacts with a mixture of conc. H2SO4 Conc. HNO3 to yield a mixture of ortho nitro anisole and
para nitro anisole.
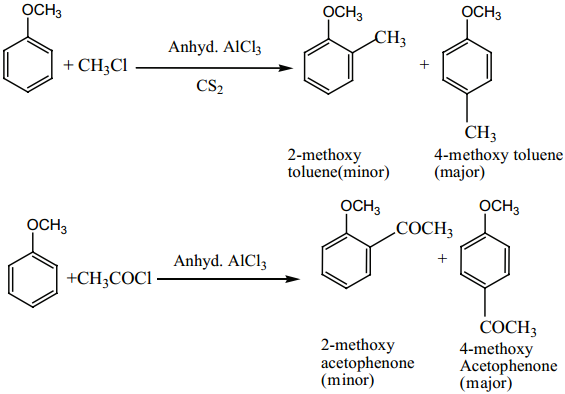
(iii) Friedel Crafts Reaction:
Anisole undergoes Fridel Craft’s reaction in presence of anhydrous AlCl3 as a catalyst.
Uses of Ethers
Uses of Diethyl Ether
- Diethyl ether is used as a surgical anaesthetic agent in surgery.
- It is a good solvent for organic reactions and extraction.
- It is used as a volatile starting fluid for diesel and gasoline engine.
- It is used as a refrigerant.
Uses of Anisole
- Anisole is a precursor to the synthesis of perfumes and insecticide pheromones,
- It is used as a pharmaceutical agent.