Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Iupac Nomenclature of Carboxylic Acids
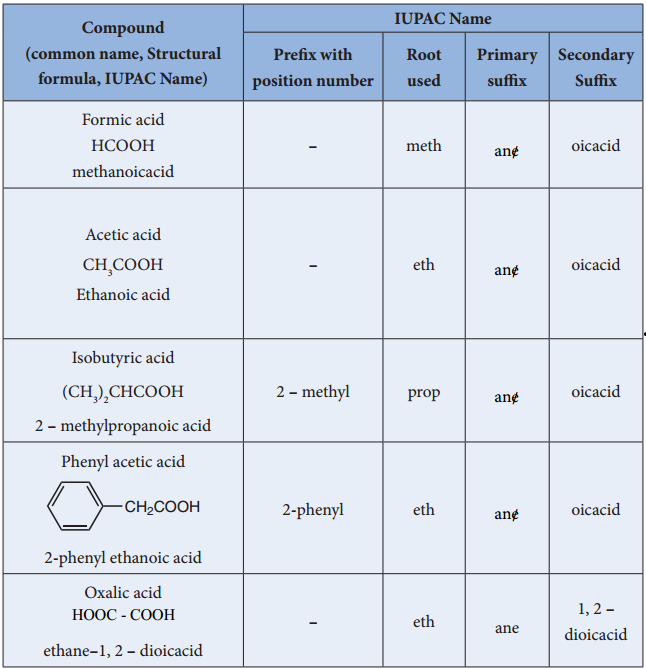
The IUPAC name of a carboxylic acid is derived from that of the longest carbon chain that contains the carboxyl group by dropping the final – e from the name of the parent alkane and adding the suffix – oic followed by the word “acid.” The chain is numbered beginning with the carbon of the carboxyl group.
Carboxylic acids are named by counting the number of carbons in the longest continuous chain including the carboxyl group and by replacing the suffix – ane of the corresponding alkane with – anoic acid.
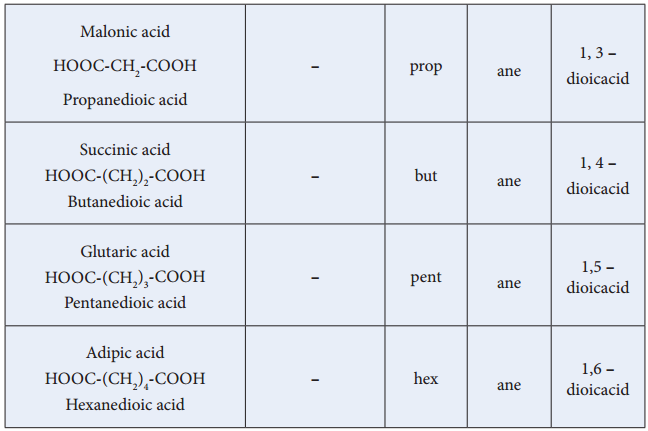
For molecules with two carboxylic acid groups the carbon chain in between the two carboxyl groups (including the carboxyl carbons) is used as the longest chain; the suffix – dioic acid is used. For molecules with more than two carboxylic acid groups, the carboxyl groups are named as carboxylic acid substituents.
Carboxylic acids are the most common type of organic acid. A carboxylic acid is an organic acid that contains a carboxyl group (C(=O)OH) attached to an R-group. The general formula of a carboxylic acid is R-COOH or R-CO2H, with R referring to the alkyl, alkenyl, aryl, or other group.
Carboxylic acids are commonly identified by their trivial names. They often have the suffix – ic acid. IUPAC-recommended names also exist; in this system, carboxylic acids have an -oic acid suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines.
Carboxylic acids occur in many common household items.
- Vinegar contains acetic acid
- Aspirin is acetylsalicylic acid
- Vitamin C is ascorbic acid
- Lemons contain citric acid, and
- Spinach contains oxalic acid.
Carboxylic acids are weak acids because they only partially ionise in solution. Their solutions do not contain many hydrogen ions compared to a solution of a strong acid at the same concentration.
Carboxylic acids are very important biologically. The drug aspirin is a carboxylic acid, and some people are sensitive to its acidity. Carboxylic acids that have very long chains of carbon atoms attached to them are called fatty acids. As their name suggests, they are important in the formation of fat in the body.
Carboxylic acids are soluble in water. Carboxylic acids do not dimerise in water, but forms hydrogen bonds with water. Carboxylic acids are polar and due to the presence of the hydroxyl in the carboxyl group, they are able to form hydrogen bonds with water molecules.
Aspirin is both an aromatic carboxylic acid (red oval) and a phenyl ester of acetic acid (blue oval). While esterification will convert the carboxylic acid group to a methyl ester, transesterification (exchange of one alcohol portion of an ester for another alcohol) to afford methyl acetate 4 and methyl salicylate 3.
A carboxylic acid is an organic compound that contains a carboxyl group (C(=O)OH) attached to an R-group. The general formula of a carboxylic acid is R-COOH, with R referring to the alkyl group. Important examples include the amino acids and fatty acids.