In this page, we are providing Matter in Our Surroundings Class 9 Extra Questions and Answers Science Chapter 1 pdf download. NCERT Extra Questions for Class 9 Science Chapter 1 Matter in Our Surroundings with Answers will help to score more marks in your CBSE Board Exams. https://ncertmcq.com/extra-questions-for-class-9-science/
Class 9 Science Chapter 1 Extra Questions and Answers Matter in Our Surroundings
Extra Questions for Class 9 Science Chapter 1 Matter in Our Surroundings with Answers Solutions
Matter in Our Surroundings Class 9 Extra Questions Very Short Answer Type
Matter In Our Surroundings Class 9 Extra Questions With Answers Question 1.
Define the term ‘matter’.
Answer:
Matter is defined as anything that occupies some space and has definite mass.
Class 9 Science Chapter 1 Extra Questions And Answers Question 2.
What is Law of Conservation of Mass?
Answer:
Law of Conservation of Mass states that mass can neither be created nor destroyed in a chemical reaction.
Matter In Our Surroundings Extra Questions Class 9 Question 3.
Define melting point.
Answer:
It is the temperature at which a solid becomes liquid at atmospheric pressure by absorbing heat.
Class 9 Chemistry Chapter 1 Extra Questions Class 9 Question 4.
Out of water and alcohol, which is more volatile?
Answer:
The boiling point of alcohol (78°C or 351K) is lower than that of water (100°C or 373K), therefore, alcohol is more volatile than water.
Matter In Our Surroundings Class 9 Extra Questions With Answers Pdf Question 5.
What is sublimation?
Answer:
Direct conversion of a solid into vapour and vice-versa (i.e., vapour into solid) is called sublimation.
Matter In Our Surroundings Class 9 Extra Questions Question 6.
Is dry ice the same thing as ordinary ice?
Answer:
No, dry ice is solid carbon dioxide while ordinary ice is solid water.
Extra Questions For Class 9 Science Chapter 1 Question 7.
Define latent heat of fusion.
Answer:
It is the heat energy required to convert 1 kg of solid into liquid at its melting point at atmospheric pressure.
Class 9 Science Ch 1 Extra Questions Question 8.
Define vapourisation.
Answer:
The process of change from liquid state to gaseous (vapour) state is called vapourisation.
Class 9 Chapter 1 Science Extra Questions Question 9.
Give the important properties on the basis of which the three states of matter can be distinguished.
Answer:
The three states of matter can distinguished on the basis of shape, volume, compressibility, packing of molecules, number of free surfaces, etc.
Ncert Class 9 Science Chapter 1 Extra Questions Question 10.
Name the term used for the solid which is directly formed from the gas.
Answer:
Sublimate.
Science Class 9 Chapter 1 Extra Questions Question 11.
Define the term volatile liquid.
Answer:
Those liquids which can change into vapour easily are termed as volatile liquids.
Ch 1 Science Class 9 Extra Questions Question 12.
What is the effect of pressure on boiling point?
Answer:
Boiling point increases with increase in pressure.
Class 9 Science Chapter 1 Extra Questions Question 13.
Name any two substances which sublime.
Answer:
Camphor, napthalene, iodine, ammonium chloride.
Class 9 Science Chapter 1 Short Question Answer Question 14.
Define condensation.
Answer:
The change of a gaseous state to a liquid state on cooling is known as condensation.
Chapter 1 Science Class 9 Extra Questions Question 15.
State the effect of surface area on rate of evaporation.
Answer:
If the surface area is increased, the rate of evaporation increases.
Question 16.
Define evaporation.
Answer:
Evaporation is a physical process in which a liquid changes to its gaseous state, at a temperature lower than its boiling point.
Question 17.
What are the ways in which a gas can be liquefied?
Answer:
Applying pressure and reducing temperature can liquefy gases.
Question 18.
What is plasma?
Answer:
It is a state of matter which consists of super energetic and super excited particles. These particles are in the form of ionised gases.
Question 19.
How do solids, liquids and gases differ in shape and volume?
Answer:
Solids have a definite shape and a fixed volume, liquids have a definite volume but no fixed shape while gases neither have a definite volume nor a definite shape.
Question 20.
Kelvin scale of temperature is regarded as better scale than Celsius. Why?
Answer:
As it has a wide range of measurement and temperature in kelvin scale always has a positive sign, hence regarded as better scale than Celsius.
Matter in Our Surroundings Class 9 Extra Questions Short Answer Type 1
Question 1.
What are characteristics of particles of matter?
Answer:
The particles of matter have following characteristics:
- Particles of matter are very very small.
- Particles of matter have space between them.
- Particles of matter attract each other.
- Particles of matter are constantly moving.
Question 2.
Write four main characteristics of solid state of matter.
Answer:
- Solids have definite mass, volume and shape.
- The particles in solid state are closely packed and empty spaces in them are negligible.
- Solids are rigid.
- Solids can have a number of free surfaces.
Question 3.
Write four main characteristics of liquid state of matter.
Answer:
- Liquids have a definite mass and volume.
- A liquid can take the shape of a container.
- Liquids have only one free surface.
- Liquids show the property of diffusion.
Question 4.
Write four characteristics of gaseous state of matter.
Answer:
- A gas has definite mass but it has neither definite shape nor definite volume.
- Gases can occupy the whole of the space available to them.
- There are larger vacant spaces between the molecules of a gas.
- Gases are highly compressible.
Question 5.
Explain evaporation and its cooling effect in terms of kinetic energy of particles.
Answer:
During evaporation, the molecules which possess higher kinetic energy leave the liquid and go into the space above the liquid as vapour. The remaining molecules possessing lower kinetic energy are left in the liquid state. Consequently, the average kinetic energy decreases which results in the fall of temperature of the liquid.
Question 6.
How is heat transferred when a solid sublimes?
Answer:
Certain solids like iodine, naphthalene, solid CO2 sublimes on heating. Heat is absorbed by the molecules of these solids rapidly which provides enough kinetic energy to show phase change into gaseous state.
Question 7.
Why do gases diffuse rapidly?
Answer:
Gases diffuse rapidly due to high speed of the particles and large space between them.
Question 8.
For any substance, why does the temperature remain constant during the change of state?
Answer:
On increasing the temperature of a substance, for example a solid, the kinetic energy of the particles increases which is used to overcome the forces of attraction between the particles therefore the temperature remains constant during the change of state.
Question 9.
Explain compressibility in gases with an example.
Answer:
Liquefied petroleum gas (LPG) cylinders are used in our homes for cooking, contains gases in the compressed state. Similarly, compressed natural gas (CNG) is used as a fuel in vehicles. Large volume of gases can be compressed in small cylinders and are transported to distant places.
Question 10.
Why solids cannot be compressed like gases?
Answer:
The particles in solids are so tightly packed that there are no or little interparticle spaces left among them. Therefore solids are not compressible like gases. Gases which have large interparticle spaces are therefore compressible.
Question 11.
Define boiling. Why is boiling considered as bulk phenomenon?
Answer:
Rapid and breaking of bubbles in the bulk of a liquid being heated is called boiling. During boiling particles from the bulk of liquid gain enough energy to get converted to vapour. Therefore it is a bulk phenomenon.
Question 12.
Why do we see water droplets on the outer surface of a glass containing ice-cold water?
Answer:
The water vapour present in air, on coming in contact with the cold glass of water, loses energy and gets converted to liquid state, which we see as water droplets.
Question 13.
Why do we sprinkle water on the roof or open ground in summer?
Answer:
During hot summer evenings, we often sprinkle water on the roof of the house or open ground in front of our house. The water evaporates by absorbing heat from the ground and the surrounding air. By losing heat, the ground becomes cool and we feel comfortable.
Question 14.
Why is ice rubbed on a burnt part of the skin?
Answer:
When a finger or some part of our body gets burnt, we rub the burnt portion with an ice cube. The reason being that due to burning, the temperature of the injured skin increases. When ice is rubbed, the excess heat from the skin is taken away by large latent heat of fusion of water. As a result, the temperature of the injured skin decreases and we feel less pain.
Question 15.
How will you demonstrate that particles of matter are continuously moving?
Answer:
When an incense stick is lit in one corner of a room, we get the smell while sitting at a distance from the stick. This is because the particles of matter are continuously moving. Because of their random motion, the particles of incense mix with the particles of air rapidly and the smell of the incense reaches us even when we are sitting at a distance from the incense stick.
Question 16.
Why do solids expand a bit on heating and contract a bit on cooling?
Answer:
The solid molecules do not have sufficient intermolecular (or interparticle) space thus its expands a bit on heating. The interparticle forces of attraction are very strong which do not let solid particles leave their mean positions. Therefore solid contracts a bit on cooling.
Question 17.
Draw diagram to shown interconversion among states of matter.
Answer:
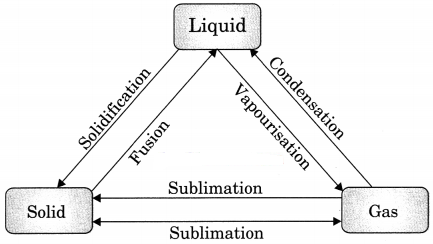
Question 18.
Why is light not considered matter?
Answer:
Matter occupies space and has mass. Light has neither of the two and that is why it is not considered as matter. It is considers as a form of energy and electromagnetic radiation.
Question 19.
Convert the following temperatures:
(a) – 78.0°C to kelvin
(b) 775 K to °C
(c) 489 K to °C
(d) 24°C to kelvin
Answer:
(a) – 78 + 273 = 195 K
(b) 775 – 273 = 502°C
(c) 489-273 = 216°C
(d) 24 + 273 = 297 K
Question 20.
Mention the difference between gas and vapour.
Answer:
Gas – The gas is a substance which exists in the gaseous state at a temperature equal to or more than the boiling point of its liquid state. For example oxygen, hydrogen, nitrogen, etc.
Vapour – A vapour is a substance which exists in the gaseous state such that its temperature is lower than that of boiling point of its liquid state. For example, water vapour, iodine vapour, etc.
Question 21.
A sample of water under study was found to boil at 102″C at normal temperature and pressure. Is the water pure? Will this water freeze at 0°C? Comment.
Answer:
It’s freezing point will be below 0°C due to the presence of a non-volatile impurity in it.
Question 22.
Water as ice has a cooling effect, whereas water as steam may cause severe burns. Explain these observations. [NCERT Exemplar]
Answer:
In case of ice, the water molecules have low energy while in the case of steam the water molecules have high energy. The high energy of water molecules in steam is transformed as heat and may cause burns. On the other hand, in case of ice, the water molecules take energy from the body and thus gave a cooling effect.
Question 23.
It is a hot summer day, Priyanshi and Ali are wearing cotton and nylon clothes respectively. Who do you think would be more comfortable and why? [NCERT Exemplar]
Answer:
Cotton being a better absorber of water than nylon helps in absorption of sweat followed by evaporation which leads to cooling. So Priyanshi would be more comfortable than Ali.
Question 24.
You want to wear your favourite shirt to a party, but the problem is that it is still wet after a wash. What steps would you take to dry it faster? [NCERT Exemplar]
Answer:
Conditions that can increase the rate of evaporation of water are:
- An increase of surface area by spreading the shirt
- An increase in temperature by putting the shirt under the Sun
- An increase the wind speed by spreading it under the fan.
Matter in Our Surroundings Class 9 Extra Questions Short Answer Type 2
Question 1.
What is evaporation? Why does evaporation cause cooling?
Answer:
The process in which a liquid changes into its vapour state at a temperature below the boiling point is called evaporation. Evaporation is an endothermic process i.e., the liquid absorbs heat during evaporation. This heat may be provided either by the surrounding or by the liquid itself. When the evaporating liquid takes the required heat from other parts of the liquid, the rest of the liquid cools down.
On the other hand, if the liquid takes heat from the surroundings, it causes cooling of the surroundings. For example, on a hot day (sunny day) we perspire. When this sweat evaporates, it absorbs the required heat from our body, and we feel cool.
Question 2.
What factors affect the rate of evaporation?
Answer:
Factors that affect the rate of evaporation are:
- Temperature: Evaporation increases with increase in temperature.
- Humidity: Evaporation decreases with an increase in humidity.
- Wind speed: Evaporation increases with an increase in wind speed.
Question 3.
What is a dry ice and what are its properties?
Answer:
Solid carbon dioxide is known as dry ice. It is stored under high pressure. Solid CO2 gets converted directly to gaseous state on decrease of pressure to 1 atmosphere without passing through the liquid state (i.e., sublimes). This is the reason that solid carbon dioxide is also known as dry ice.
It is mainly used as a cooling agent because its temperature is very low than ice formed from water. Dry ice is commonly used in theaters and in movies to produce the effect of fog.
Question 4.
Why should we wear cotton clothes in summer?
Answer:
During summer, we perspire more because of the mechanism of our body which keeps us cool. We know that during evaporation, the particles at the surface of the liquid gain energy from the surroundings or body surface and change into vapour. The heat energy equal to latent heat of vapourisation is absorbed from the body leaving the body cool. Cotton, being a good absorber of water helps in absorbing the sweat and exposing it to the atmosphere for easy evaporation.
Question 5.
Give the main postulates of kinetic theory of matter.
Answer:
The main postulates of kinetic theory are:
- All matter is made up of a large number of extremely small particle called molecules.
- The molecules are always in a state of rapid random motion.
- The molecules possess kinetic energy.
- There are attractive forces between the molecules.
- The kinetic energy of molecules increases with increase in temperature.
Question 6.
Identify each of the following changes of state as evaporation, boiling or condensation. Give reason for your answer.
(a) Wet clothes dry when spread on wire.
(b) After a hot shower, your bathroom mirror is covered with water.
(c) Lava flows into the ocean and forms steam.
Answer:
(a) Evaporation, because conversion of liquid water to vapour occur at room temperature.
(b) Condensation, because hot water vapour condense to form liquid water.
(c) Boiling, because heat of lava makes liquid water boil and hence steam is formed.
Question 7.
Why do surgeons often spray some ether on the skin before performing minor surgery?
Answer:
Quite often doctors spray ether on a portion of skin the before performing minor surgery. The reason being that a ether has very low boiling point (308 K). Therefore, it evaporates quite rapidly. The heat energy needed for evaporation is taken from the skin. As a result, the temperature of the skin becomes so low that it almost becomes numb. Due to this numbness, the patient does not feel much pain when a minor cut is made in the skin in order to perform surgery.
Similarly, when a player gets injured during a game, ethyl chloride on the injured portion of the body. Since the boiling point of ethyl chloiide (285.5K or 12.5°C) is very low, it quickly evaporates. The heat energy needed for evaporation is taken from the skin. By losing heat, temperature of the skin becomes so low that it almost becomes numb. Due to this numbness, the player does not feel much pain.
Question 8.
Comment upon the following:
(i) Rigidity
(ii) Compressibility
(iii) Fluidity
Answer:
(i) Rigidity means tendency to maintain shape when some outside force is applied due to strong interparticle force.
(ii) Compressibility means tendency to decrease volume when some outside force is applied. Due to large interparticle distances in gases their volume decreases when some pressure is applied on them therefore, gases possess high compressibility.
(iii) Fluidity means tendency to flow. Due to large interparticle distances and weak forces of attraction gases have highest fluidity.
Question 9.
Comment on the following statements:
(а) Evaporation produces cooling.
(b) Rate of evaporation of an aqueous solution decreases with increase in humidity.
(c) Sponge though compressible is a solid. [NCERT Exemplar]
Answer:
(а) For evaporation to occur, heat energy is needed. This heat energy is taken out from the substance or the surroundings. As a result surrounding becomes cool. Thus, evaporation causes cooling.
(b) By humidity we mean, the amount of water vapours present in the air. With increase in humidity the rate of evaporation decreases. If the humidity of air is already high, it can hold only a little more amount of water vapour to reach that optimum level, therefore the rate of evaporation decreases.
(c) Sponge has large number of minute holes in which air is trapped. When we press it, air expelled and sponge is compressed to a small amount of matter which has a definite shape as well as definite volume.
Matter in Our Surroundings Class 9 Extra Questions Long Answer Type
Question 1.
List any five physical properties of liquids.
Answer:
- Liquids do not have fixed shape or boundaries.
- They have fixed volume.
- They exhibit fluidity i.e., they can flow.
- Less compressible as compared to gases but higher than solids.
- Lower density as compared to solids.
- Compared to solids, liquids have higher kinetic energy but less than gases.
- The intermolecular forces of attraction are weaker than those of solids.
- Show the property of intermixing i.e., can diffuse.
Question 2.
Fill in the blanks:
(а) Evaporation of a liquid at room temperature leads to a ………………… effect.
(b) At room temperature the forces of attraction between the particles of solid substances are ………………… than those which exist in the gaseous state.
(c) The arrangement of particles is less ordered in the state. However, there is no order in ………………… the state.
(d) is the change of gaseous state directly to solid state without going through the ………………… state.
(e) The phenomenon of change of a liquid into the gaseous state at any temperature below its boiling point is called ………………… [NCERT Exemplar]
Answer:
(a) cooling
(b) stronger
(c) liquid, gaseous
(d) Sublimation, liquid
(e) evaporation
Question 3.
Classify the following into osmosis/diffusion [NCERT Exemplar]
(a) Swelling up of a raisin on keeping in water.
(b) Spreading of virus on sneezing.
(c) Earthworm dying on coming in contact with common salt.
(d) Shrinking of grapes kept in thick sugar syrup.
(e) Preserving pickles in salt.
Answer:
(a) Osmosis
(b) Diffusion
(c) Osmosis
(d) Osmosis
(e) Osmosis
Question 4.
You are provided with a mixture of naphthalene and ammonium chloride by your teacher. Suggest an activity to separate them with well labelled diagram. [NCERT Exemplar]
Answer:
Naphthalene is insoluble in water but soluble in ether an organic solvent. It is volatile at room temperature. Ammonium chloride is soluble in water and volatile at higher temperature. It decomposes on heating to dryness.
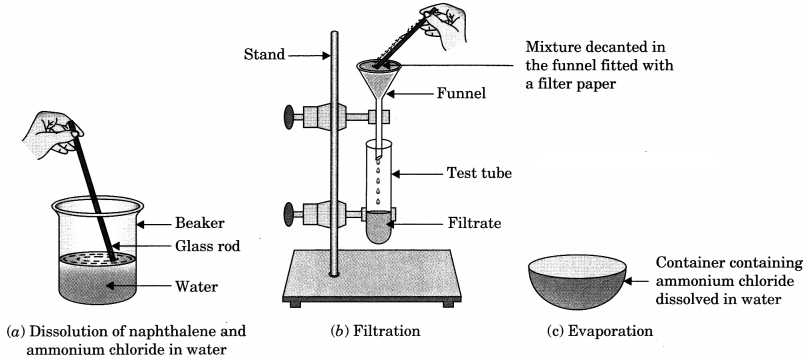
Question 5.
Why does the temperature of a substance remain constant during its melting point or boiling point?
Answer:
The temperature of a substance remains constant at its melting and boiling points untill all the substance melts or boils because, the heat supplied is continuously used up in changing the state of the substance by overcoming the forces of attraction between the particles. This heat energy absorbed without showing any rise in temperature is given the name latent heat of fusion/latent heat of vapourisation.
Matter in Our Surroundings Class 9 Extra Questions HOTS
Question 1.
Arrange the following substances in increasing order of force of attraction between the particles.
(a) Milk
(b) Salt
(c) Oxygen
Answer:
Oxygen < Milk < Salt.
Question 2.
Explain with an experiment to show gases do not have fixed shape or volume.
Answer:
Experiment: To show gases do not have fixed shape or volume.
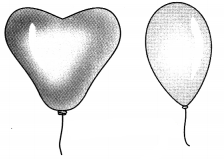
Method:
- Take two balloons of different shapes. For example, one round and one heart shape or cylindrical.
- Fill the balloons with air.
Observation: Air takes up the shape of balloon.
Conclusion: This shows air has no definite shape or volume. It takes up the shape of the balloon.
Question 3.
Name the change of state during the following changes:
(a) Drying of wet clothes
(b) Melting of wax
(c) Melting of ice
(d) Formation of clouds
Answer:
(a) Liquid to gaseous state
(b) Solid to liquid state
(c) Solid to liquid state
(d) Liquid to gaseous state
Question 4.
With proper explanation, explain whether the following statements are true or false?
(a) Sublimation occurs only when the solid is heated.
(b) A lighter gas can move downwards and a heavier gas can move upwards.
(c) Interconversion of matter is a constant temperature process.
Answer:
(a) Statement is wrong. Sublimation may occur on its own or by heating, e.g., camphor, naphthalene, iodine, etc., sublime slowly at room temperature.
(b) Statement is true. Diffusion occurs against the law of gravitation. Therefore, lighter gases can also diffuse downwards and the heavier gases can also diffuse upwards. However rate of diffusion of lighter gases is faster than those of heavier gases.
(c) Statement is true. During interconversion of state of matter from solid to liquid or from liquid to gas, it tends to reach its melting point or boiling point. At this point, the temperature remains constant unit it has changed in another state.
Question 5.
What is meant by Bose-Einstein Condense?
Answer:
(a) In 1920, Indian scientist Satyendra Nath Bose did some calculations, based on which Albert Einstein predicted that a new state of matter should exist.
(b) This new state was named as Bose-Einstein Condensate (BEC). In 2001, Cornell, Ketterie and Wieman of USA received Noble Prize for actually making this state in laboratory. BEC is made by cooling gas of very low density to super low temperature.
Question 6.
A student heats a beaker containing ice and water. He measures the temperature of the content of the beaker as a function of time. Which of the following figure would correctly represent the result? Justify your choice. [NCERT Exemplar]
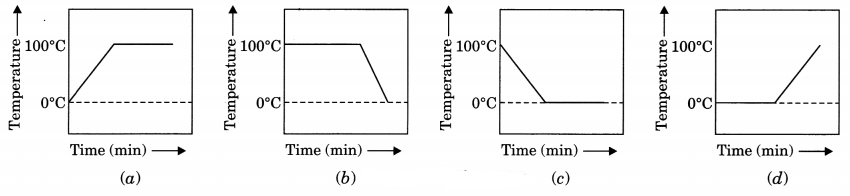
Answer:
Since ice and water are in equilibrium, the temperature would be zero. When we heat the mixture, energy supplied is utilised in melting the ice and the temperature does not change till all the ice melts because of latent heat of fusion. On further heating, the temperature of the water would increase. Therefore the correct option is (d).
Question 7.
Look at the figure and suggest in which of the vessels A, B, C or D the rate of evaporation will be the highest? Explain. [NCERT Exemplar]
Answer:
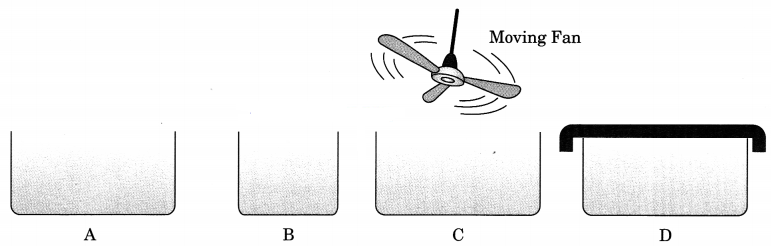
(c) The rate of evaporation increases with an increase in the surface area of absorption because evapo-ration in a surface phenomenon. Also, with the increase in air speed, the particles of water vapour will move away with the air, which will increase the rate of evaporation.
Matter in Our Surroundings Class 9 Extra Questions Value Based (VBQs)
Question 1.
In a hot summer day, Rajeev wants to watch a movie in a nearest cineplex. His mother wears a nylon saree so Rajeev suggest her to wear cotton saree.
(i) Why does Rajeev suggest her to wear cotton saree and not nylon saree?
(ii) Mention the values exhibited by Rajeev.
Answer:
(i) On a hot summer day, we sweat a lot. Cotton clothes absorb sweat from the body. As the sweat evaporates it results in cooling giving comfort to the body.
(ii) Caring, use of knowledge of science.
Question 2.
Mohan was getting late for school. He tried to sip tea from the cup. His father advised him to use a plate and asked him to sip the tea from the plate. Mohan followed the advice and finished his tea very easily.
(i) Why is siping off tea easier from a plate?
(ii) Mention the values exhibited by Mohan’s father.
Answer:
(i) A plate has a larger surface area than a cup. Evaporation becomes faster in this case. Since cooling is always caused during evaporation the temperature got lowered. Therefore it became easier to sip tea from a plate.
(ii) Caring, use of knowledge of science, helpful.
