 On this page, you will find Metals and Non-metals Class 10 Notes Science Chapter 3 Pdf free download. CBSE NCERT Class 10 Science Notes Chapter 3 Metals and Non-metals will seemingly help them to revise the important concepts in less time.
On this page, you will find Metals and Non-metals Class 10 Notes Science Chapter 3 Pdf free download. CBSE NCERT Class 10 Science Notes Chapter 3 Metals and Non-metals will seemingly help them to revise the important concepts in less time.
CBSE Class 10 Science Chapter 3 Notes Metals and Non-metals
Metals and Non-metals Class 10 Notes Understanding the Lesson
1. Element is the simplest form of matter which contains one kind of atoms. About 118 elements are known today. There are more than 90 metals, 22 non-metals and a few metalloids.
- Sodium (Na), potassium (K), magnesium (Mg), aluminium (Al), calcium (Ca), Iron (Fe), Barium (Ba) are some metals.
- Oxygen (O), hydrogen (H), nitrogen (N), sulphur (S), phosphorus (P), fluorine (F), chlorine (Cl), bromine (Br), iodine (I) are some non-metals.
2. Physical Properties of Metals
- Metals in their pure state, have a shining surface. This property is called metallic lustre.
- Metals can be beaten into thin sheets. This property is called Gold and silver are the most malleable metals.
- Metals have ability to be drawn into thin wires. This property is called ductility. Gold is the most ductile metal.
- Metals are good conductors of heat and have high melting points. The best conductor of heat are silver and copper. Lead and mercury are comparatively poor conductors of heat.
- The metals that produce a sound on striking a hard surface are said to be
- Alkali metal (Li, Na, K) are so soft that they can be cut with a knife. They have low densities and low melting points.
- Metals have high melting point but gallium and caesium have very low melting points. These two metals will melt if you keep them on your palm.
3. Physical Properties of Non-Metals
- Non-metal are either solids or gases except bromine which is a liquid.
4. Some other exceptions:
- Iodine is a non-metal but it is lustrous.
- Carbon is a non-metal which exist in two allotropic forms: diamond and graphite. Diamond is the hardest substance with a very high melting point. Graphite is a conductor of electricity.
5. Chemical Properties of Metals
I. All metals combine with oxygen to form metal oxides.
Metal + Oxygen → Metal oxide
For example,
2Cu + O2 → 2CuO
4Al + 3O2 → 2Al2O3
6. Remember
Different metals exhibit different reactivity towards oxygen. Metals such as K and Na react so vigorously that they catch fire if kept in the open. Hence, to protect them they are kept immersed in kerosene oil.
Generally metal oxides are basic in nature. But some oxides like aluminium oxide, zinc oxide show both acidic as well as basic behaviour.
Al2O3 + 6HCl → 2AlCl3 + 3H2O
Al2O3 + 2NaOH → 2NaAlO2 + H2O
Most metal oxides are insoluble in water but some of these dissolve in water to form alkalies. Sodium oxide and potassium oxide dissolve in water to produce alkali as follows:
Na2O(s) + H2O(l) → 2NaOH(aq)
K2O(s) + H2O(l) → 2KOH(aq)
II. Metals react with water and produce a metal oxide and hydrogen gas. Metal oxides that are soluble in water dissolve in it further to form metal hydroxide. But all metals do not react with water.
Metal + Water → Metal Oxide + Hydrogen
Metal Oxide + Water → Metal hydroxide
Potassium and sodium react violently with cold water. The reaction is exothermic, so the released hydrogen catches fire immediately.
2K(s) + 2H2O(l) → 2KOH(aq) + H2(g) + Heat
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g) + Heat
The reaction of calcium with water is less violent. The heat evolved is not sufficient for the hydrogen to catch fire.
Ca(s) + 2H2O(l) → Ca(OH)2(aq) + H2(g)
Mg(s) + 2H2O(l) → Mg(OH)2(aq) + H2(g)
- Calcium starts floating because the bubbles of hydrogen gas formed stick to the surface of the metal.
- Magnesium does not react with cold water. It reacts with hot water to form magnesium hydroxide and hydrogen.
Aluminium, iron and zinc do not react either with cold or hot water. But they react with steam to form the metal oxide and hydrogen.
2Al(s) + 3H2O(g) → Al2O3(s) + 3H2(g)
3Fe(s) + 4H2O(g) → Fe3O4(s) + 4H2(g)
Lead, copper, silver and gold do not react with water at all.
III. Metals react with acids to give a salt and hydrogen gas.
Metal + Dilute acid → Salt + Hydrogen
- Hydrogen gas is not evolved when a metal reacts with HNO3. It is because HNO3 is a strong oxidising agent. It oxidises the H2 produced to water and itself get reduced to any of the nitrogen oxides.
- Magnesium (Mg) and manganese (Mn) react with very dilute HNO3 to evolve H2
- Copper does not react with dilute HCl.
IV. Reactive metals can displace less reactive metals from their compounds in solution or molten form.
Metal A + Salt solution of B → Salt solution of A + Metal B
Fe + CuSO4 → FeSO4 + Cu
Zn + CuSO4 → ZnSO4 + Cu
Reactivity of metal can be explained on the basis of displacement reactions
7. Knowledge Plus
Aqua regia (Latin for ‘royal water) is a freshly prepared mixture of concentrated HCl and concentrated nitric acid in the ratio of 3:1. It is a highly corrosive, fuming liquid and dissolve gold and platinum.
8. The Reactivity Series
The reactivity series is a list of metals arranged in the order of their decreasing activities.
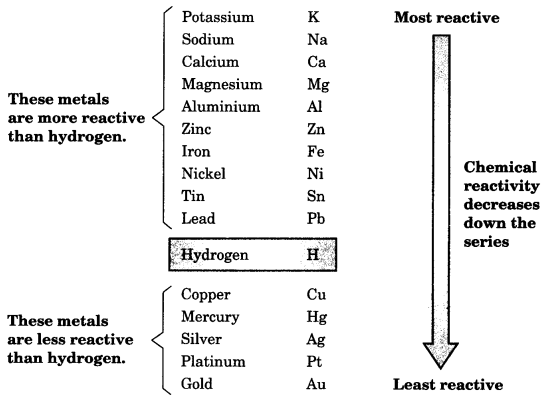
Metals occupying higher position in the series have more tendency to lose electrons and are more reactive. The metals at the bottom of the series are least reactive. Thus, potassium is the most reactive metal.
9. How do metals and non-metals react?
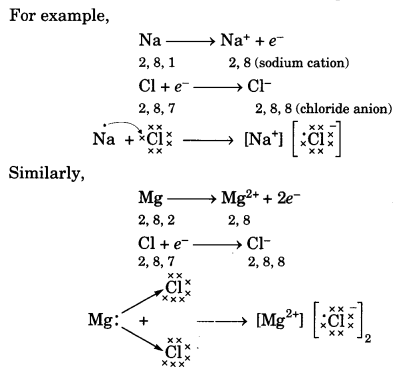
Reactivity is the tendency of elements (metals and non-metals) to attain a completely filled valence shell. Metal atoms having 1, 2 or 3 electrons in their outermost shell can lose electrons to non-metal atoms having 5,6 or 7 electrons to attain the electronic configuratìoñ of the nearest noble gas (i.e., completely filled valence shell).
Thus the metal atom becomes a positively charged ion or cation and the non-metal atom becomes a negatively charged ion or anion. The cation and anion being oppositely charged attract each other and are held by strong electrostatic forces of attraction to exist as an ionic compound. For example,
Metal ore heated strongly in limited or no supply of air (Calcination).
![]()
10. Reduction of Metal Oxide:
(i) Using coke: Coke as a reducing agent.
![]()
(ii) Using displacement reaction: Highly reactive metals like Na, Ca and A1 are used to displace metals of lower reactivity from their compounds. These displacement reactions are highly exothermic.
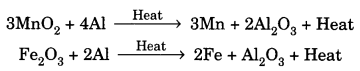
11. Thermite Reaction: Reduction of a metal oxide to form metal by using aluminium powder as a reducing agent. This process is used to join broken pieces of heavy iron objects or welding.
12. Extracting Metals at the Top of the Activity Series:
- These metals have more affinity for oxygen than carbon so they cannot be obtained from their compounds by reducing with carbon.
- They are obtained by electrolytic reduction, for example, Sodium is obtained by electrolysis of its molten chloride NaCl→ Na+ + Cl–
As electricity is passed through the solution, metal gets deposited at the cathode and non-metal at the anode.
- At cathode: Na+ + e– →Na
- At anode: 2Cl– →Cl2 (g) + 2e–
III. Refining of Metals
- Impurities present in the obtained metal can be removed by electrolytic refining.
Copper is obtained using this method. Following are present inside the electrolytic tank.
- Anode – slab of impure copper
- Cathode – slab of pure copper
- Solution – aqueous solution of copper sulphate with some dilute sulphuric acid.
- From anode, copper ions are released in the solution and equivalent amount of copper from solution is deposited at cathode.
- Insoluble impurities containing silver and gold gets deposited at the bottom of anode as anode mud.
13. Corrosion
- Metals are attacked by substances in the surroundings like moisture and acids.
- Silver—It reacts with sulphur in air to form a black coating of silver sulphide.
- Copper—It reacts with moist carbon dioxide in air and forms a green coating of copper carbonate.
- Iron—acquires a coating of a brown flaky substance called rust. Both air and moisture are necessary for rusting of iron. Rust is hydrated Iron (III) oxide e., Fe2O3.xH2O
14. Prevention of Corrosion
- Rusting of iron is prevented by painting, oiling, greasing, galvanizing, chrome plating, anodising and making alloys.
- In galvanization, iron or steel is coated with a thin layer of zinc. Zinc oxide formed due to oxidation is impervious to air and moisture protecting further layers from corrosion.
15. Alloys: These are homogeneous mixture of metals with metals or non-metals.
Adding small amount of carbon makes iron hard and strong.
| Name of Alloy | Properties | Constituent metal/ Non-metal |
| 1. Steel | Hard | Iron and carbon |
| 2. Stainless steel | Hard, rust free | Iron, nickel and chromium |
| 3. Brass | Low electrical conductivity than pure metal | Copper and zinc |
| 4. Bronze | Hard and easily cast | Copper and tin |
| 5. Solder | Low MP, used to weld wires | Lead and tin |
| 6. Amalgam | Used by dentists | Mercury and any other metal |
Class 10 Science Chapter 3 Notes Important Terms
Corrosion is the eating up of metals by the action of air, moisture or a chemical on their surface.
Rust is mainly hydrated iron (III) oxide Fe2O3.xH2O due to corrosion.
Ores are the minerals from which metals can be extracted conveniently and profitably.
Minerals are natural materials in which the metals or their compounds are found in the Earth.
Covalent bond is the chemical bond formed by sharing of electrons between two atoms. Aqua-regia is a freshly prepared mixture of 1 part of concentrated nitric acid and 3 parts of concentrated hydrochloric acid.
Brass is an alloy of copper and zinc. Bronze is an alloy of copper and tin.
Metallurgy is the process of extraction of a metal from its ore and its refining.
Activity series is the arrangement of metals in the order of decreasing reactivity.