NCERT Exemplar Solutions for Class 11 Biology Chapter 9 Biomolecules
These Solutions are part of NCERT Exemplar Solutions for Class 11 Biology. Here we have given NCERT Exemplar Solutions for Class 11 Biology Chapter 9 Biomolecules.
VERY SHORT ANSWER QUESTIONS
Question 1.
Medicines are either man made (i.e. synthetic) or obtained from living organisms like plants, bacteria, animals, etc., and hence, the latter are called natural products. Sometimes, natural products are chemically altered by man to reduce toxicity or side effects. Write against each of the following whether they were initially obtained as a natural product or as 3 synthetic chemical.
(a) Penicillin
(b) Sulphonamide
(c) Vitamin-C
(d) Growth hormone
Solution:
(a) Penicillin is a group oT antibiotics derived from fungi Penicillium obtained naturally.
(b) Sulphonamide an antimirobial agent is a synthetic chemical.
(c) Vitamin-C or L-ascorbic acid or ascorbate is a natural product and an essential nutrient for humans. It is present in citrus fruits.
(d) Growth hormone also known as somatotropin or somatropin is a peptide hormone occurring naturally in the body it stimulates growth.
Question 2.
Write the name of any one amino acid, sugar, nucleotide and fatty acid.
Solution:
(a) Amino acid — Leucine
(b) Sugar — Lactose
(c) Nucleotide — Adenosine triphosphate
(d) Fatty acid — Palmitic acid
Question 3.
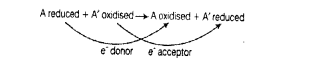
Reaction given below is catalysed by oxidoreductase between two substrates A and
A’, complete the reaction.
A reduced + A’ oxidised —>
Solution:
Oxidoreductase is an enzyme that catalyses oxidation reduction reactions. This enzyme is associated in catalysing the transfer of electron from one molecule (the reduction), also called as electron donor, to another molecule (the oxidant), also called as electron acceptor.
The complete reaction is
Question 4.
How are prosthetic groups different from co¬factors?
Solution:
Organic compounds that are tightly bound to the apoenzyme, (an enzyme without cofactor) by covalent or non-covalent bonds are prosthetic groups e.g., peroxidase and
catalase catalyse the breakdown of hydrogen peroxide to water and oxygen where haeme is the prosthetic group and it is a part of the active site of the enzyme.
Co-factor is small, heat stable and non-protein part of conjugate enzyme. It may be inorganic or organic in nature. Co-factors when loosely bound to an enzyme is called coenzyme and when tightly bound to apoenzyme is called prosthetic group.
Question 5.
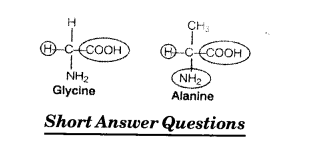
Glycine and alanine are different with respect to one substituent on the a-carbon. What are 4. the other common substituent groups?
Solution:
The common substituted groups in both the amino acids are NH2 COOH and H.
SHORT ANSWER QUESTIONS
Question 1.
Enzymes are proteins. Proteins are long chains of amino acids linked to each, other by peptide bonds. Amino acids have many functional groups in their structure.
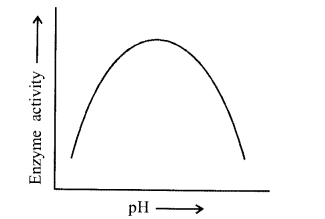
These functional groups are many of them at least, ionisable. As they are peak acids and bases in chemical nature, this ionisation is influenced by pH of the solution. For many enzymes, activity is influenced by surrounding pH. This is depicted in the curve below, explain briefly.
Solution:
Enzymes, generally function in a narrow range of pH. Most of the enzymes show their highest activity at a particular pH called optimum pH- activity declines below and above this value. Extremely high or low pH values generally results in complete loss of activity for most enzymes. The given graph represents the maximum enzyme activity at the optimum pH.
Question 2.
Explain the association of carbohydrate to the plasma membrane and its significance.
Solution:
Secondary metabolites are chemicals produced by plants which do not play any [role] in growth, photosynthesis reproduction or other primary functions of the plant. Rubber (cis 1,4- polyisopyrene) is a secondary metabolite.
(i) Rubber is extracted from Hevea brasiliensis (rubber tree)
(ii) It is a byproduct of the lactiferous tissue of the vessels that are in the form of latex.
(iii) It contains over 400 isoprene units and thus is the largest of the terpenoids.
(iv) It is elastic, water proof and a good conductor of electricity.
Question 3.
Nucleic acids exhibit secondary structure, justify with example.
Solution:
- Nucleic acids are large biological molecules, essential for all known forms of life.
- The secondary structure of a nucleic acid molecule refers to the base pairing interactions within a single molecule or set of interacting molecules.
- DNA and RNA represent two main nucleic acids, their secondary structures however differ the secondary structure of DNA comprises of two complementary strands of polydeoxyribonucleotide, spirally coiled on a common axis forming a helical structure.
- This double helical structure of DNA is stabilized by phosphodiester bonds (between 5’ of sugar of one nucleotide and 3 sugar of another nucleotide), hydrogen bonds (between bases, and ionic interactions.
Question 4.
Comment on the statement ‘living state is a non-equilibrium steady state to be able to perform work’
Solution:
- Living organism are not in equilibrium because work cannot be performed by a system at equilibrium.
- The living organisms exist in a steady state characterised by concentration of each of the biomoleculSs.
- These biomolecules are in a metabolic flux. Any chemical or physical process moves simultaneously to equilibrium.
- Living organisms work continuously and they cannot afford to reach equilibrium.
- The living state thus is an a non-equilibrium steady-state to be able to perform work. This achieved by energy input provided by metabolism.
LONG ANSWER QUESTIONS
Question 1.
What are different classes of enzymes? Explain any two with the type of reactions they catalyse.
Solution:
Enzymes are divided into six classes each with
4-13 sub-classes and named accordingly by a number comparising of four digits.
(i) Oxidoreductases/dehydrogenases : These enzymes take part in oxidation, reduction or transfer of electrons,
(ii) Transferase : These enzymes transfer a functional group (other than hydrogen).
from one molecule to another. The transfer , chemical group does not occur in free state.
(iii) Hydrolases : These enzymes catalyse the hydrolysis of bonds like ester, ether,
peptide, glycosidic C-C, C-halide, P-N etc.
![]()
(iv) Lyases cause cleavage, removal of groups without hydrolysis and addition of groups to double bonds or removal of groups producing double bonds.
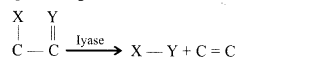
(v) Isomerases rearrangement of molecular structure to effect isomeric changes. They are of three types isomerases, epimerases and mutases.
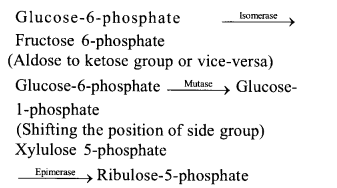
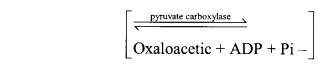
(vi) Ligases catalyse bonding of two chemicals with the help of energy obtained from ATP resulting formation of bonds such as C—O, C—S, C—N and P—O e.g., pyruvate carboxylase
Pyruvric acid + C02 + ATP + H20
We hope the NCERT Exemplar Solutions for Class 11 Biology at Work Chapter 9 Biomolecules, help you. If you have any query regarding NCERT Exemplar Solutions for Class 11 Biology at Work Chapter 9 Biomolecules, drop a comment below and we will get back to you at the earliest.