NCERT Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements
These Solutions are part of NCERT Solutions for Class 11 Chemistry. Here we have given NCERT Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements
Question 1.
Discuss the pattern of variation in oxidation states of :
(1) B to Tl
(2) C to Pb.
Answer:
(1) B to Tl. The common oxidation states are +1 and +3. The stability of
+ 3 oxidation state decreases from B to Tl.
+ 1 oxidation state increases from B to Tl.
Oxidation States. The elements of the boron family (Group 13) have ns2pl confi’guration. This means that they have three valence electrons available for bond formation. By losing these electrons, they are expected to show + 3 oxidation states in their compounds. However, the following trends are observed in the oxidation states of these elements.
- The first two elements boron and aluminium show only +3 oxidation state in the compounds but the remaining elements gallium, indium and thallium also exhibit + 1 oxidation state in addition to + 3 oxidation state i. e., they show variable oxidation states.
- The stability of +3 oxidation state decreases from aluminium onwards and in case of last element thallium, + 1 oxidation is more stable than +3 oxidation state which means that TICl is more stable than TlCl3.
Explanation. The above trend is explained with the help of the phenomenon of inert pair effect.
It represents the reluctance or inertness of the valence s-electrons of heavier elements of p-block to take part in the bond formation because of ineffective shielding of these electrons from the attraction of nucleus by the intervening d and f electrons.
As a result of the inert pair effect, the electron pair representing the valence 5-electrons is more exposed to the nucleus than the p- electrons. In other words, these are held tightly by the nucleus and are not readily available for the bond formation. However, valence p-electrons are available for the same. The inert pair effect becomes more or more predominant as we go down the group because of effect of increased nuclear charge outweighs the effect of increased atomic size. In other words, the valence 5-electrons become more and more reluctant to be available for bond formation. As a result, the valence ^-electrons will be more available accounting for + 1 oxidation state and + 3 oxidation state will not be shown by these elements so easily. The inert pair effect is maximum in the last element thallium (Tl) in boron family since it is the heaviest with maximum atomic number (Z = 81). Therefore, it shows mainly + 1 oxidation state or we can say that TICl exists while TlCl3 is rather unstable. Keeping this in mind, the relative stabilities of M + (monovalent) and M?+ (trivalent) cations follow the order:
B3+ > Al3+ > Ga3+ > In3+ > Tl3+
B+ < Al+ < Ga+ < In+ < TI+
The inert pair is not restricted only to the members of boron family. It is also present in the heavier members of group 14 (Sn and Pb) and group 15 (Sb and Bi). Please note that in the 5-block, the group 1 and 2 elements show only one oxidation state which is the same as the group valency. In the p-block, the elements have tendency to show variable oxidation states which are different from the group valencies. The elements presents in group 16 and group 17 also exhibit variable oxidation states ion not because of inert pair effect but due to the presence of vacant ri-orbitals in the valence shells of their atoms to which the electrons can be promoted from s- and p-orbitals also present in the valence shell. We shall study in detail, the variable oxidation states of these elements in the next class.
(2) C to Pb. The common oxidation states are +4 and +2. The stability of
+4 oxidation state decreases from C to Pb
+2 oxidation state increases from C to Pb.
Down the family, tendency to show +4 oxidation state decreases while +2 oxidation state increases. This is explained on the basis of inert pair As a result, the valence -electrons because of their greater penetration into the nucleus are not so easily available for bond formation or these are reluctant to participate in the bond formation while the valence -electrons are easily available. The inert pair effect is more prominent in tin and lead because of very high atomic numbers. As a result, both of them normally show + 2 oxidation states i.e., as Sn2+ and Pb2+ ions. However, if sufficient energy is available, the valence electrons also become available to account for +4 oxidation states of these elements (Sn4+ and Pb4+ ions).
Question 2.
How can you explain higher stability of BCl3 as compared to TlCl3 ?
Answer:
In case of boron (B) atom, the inert pair effect is negligible. This means that all the three valence electrons (2s2 \( { p }_{ x }^{ 1 } \) )are available for bonding with chlorine atoms. Therefore, BCl3 is quite stable. However, in case of thallium (Tl), the valence 5-electrons (6s2) are experiencing maximum inert pair effect. This means that only valence p-electron (6p1) is available for bonding. Under these circumstances, TlCl is very much stable while TlCl3 is comparatively little stable.
Question 3.
Why does BF3 behave as Lewis acid ?
Answer:
BF3 behaves as a Lewis acid because central boron atom has only six electrons (three pairs) after sharing with the electrons of the F atoms. It is an electron deficient compound and, therefore, behaves as a Lewis acid.
Boron Halides. The halides of boron are covalent in nature because the central boron atom, as stated earlier, is very small in size and cannot part with the valence electrons to give a trivalent B3+ Thus, BF3, BCl3, BBr3 and BI3 are all covalent in nature.
In these halides, the central B atom is sp2 hybridised with a vacant 2p orbital (1 .v2 2s1 2pxl2pyi2pz°). The hybridised orbitals are directed towards the three corners of an equilateral triangle and are involved in covalent bond formation with the half filled p-orbitals of the halogen atoms (ns2p5).
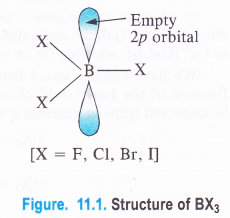
The empty 2p orbital lies perpendicular to the hybridised orbitals. Since it is empty, it can accept an electron pair from electron donor species (Lewis bases) resulting in co-ordinate or dative bond formation. With three shared pairs of electrons on the central boron atom, boron halides are Lewis acids i.e., electron deficient molecules and take part in Lewis acid – base reactions. In the compounds thus formed, boron atom undergoes a change in state of hybridisation from sp2 to sp3. These are tetrahedral in nature.
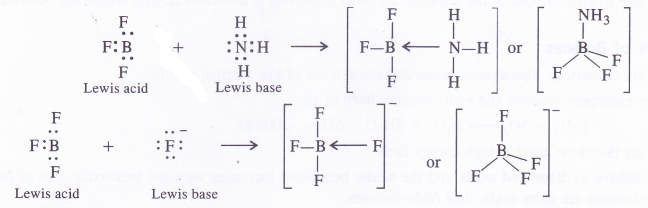
Question 4.
Consider the compounds BCl3 and CCl4, How will they behave towards water ?
Answer:
In BCl3 (B atom is sp2 hybridised), the B atom has incomplete octet and unhybridised 2p-orbital which can take up electron pair from the H2O molecule to form addition product.
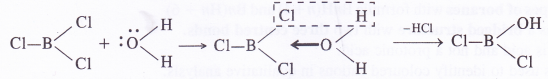
In this way, a Cl atom has been replaced by OH group on reacting with water. Similarly, the other two Cl atoms will also be replaced by the OH groups as follows :
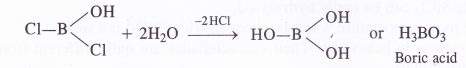
This shows that boron trichloride has undergone hydrolysis. But this is not possible with carbon tetrachloride (CCl4). The carbon atom has a complete octet and there is no scope of forming addition product with H2O molecules. As a result, carbon tetrachloride does not get hydrolysed. When added to water, it even does not mix and forms a separate oily layer.
Question 5.
Is boric acid a proton acid ? Explain.
Answer:
Boric acid is not a proton acid. It is a Lewis acid and accepts electron pair from hydroxyl ion of H2O molecule.
B(OH)3 + 2HOH →[B(OH)4]– + H3O+
Question 6.
Explain what happens when boric acid is heated ?
Answer:
Preparation of Boric Acid. Boric acid can be prepared by the following methods.
(a) From borax. A hot and concentrated solution containing borax is boiled with hydrochloric or sulphuric acid. The solution upon concentration and cooling gives crystals of boric acid.
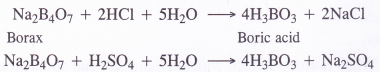
(b) From Colemanite. Sulphur dioxide gas is passed through the hot concentrated solution of mineral colemanite made in water. The solution upon concentration followed by cooling gives crystals of boric acid. Calcium bisulphite remains in solution as it is highly soluble in water.
![]()
(c) From boron compounds by hydrolysis. Certain boron compounds upon boiling with water (upon hydrolysis) give boric acid.
BCl3 + 3H2O→ H3BO3 + 3HCl
BN + 3H2O → H3BO3 + NH3
Question 7.
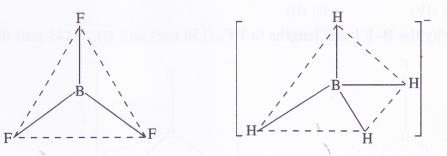
Describe the shapes of BF3 and BH4 Assign the hybridisation of boron atom in these species.
Answer:
BF3 [H] : \(\frac { 1 }{ 2 } \) [3 + 3, – 0 + 01 = \(\frac { 6 }{ 2 } \) = sp2 (trigonal planar)
BH4 [H] : \(\frac { 1 }{ 2 } \) [3 + 4 – 0 + 1] = \(\frac { 8 }{ 2 } \) = sp3 (tetrahedral)
Question 8.
Write reactions which justify amphoteric nature of aluminium.
Answer:
Aluminium can react both with acids and bases. It is therefore, amphoteric in nature. For example,
2Al (s) + 6HCl (dil) →2AlCl3 (aq) 4- 3H2 (g)
2Al (s) + 2NaOH (aq) + 6H2O (l) → 2Na+[Al(OH)4]– (aq) + 3H2 (g)
Question 9.
What are electron deficient compounds ? Are BCl3 and SiCl4 electron deficient ? Explain.
Answer:
Electron deficient compounds are the compounds in which the central atom in their molecules has urge or tendency to take up one or more electron pairs. The electron deficient compounds are also
called Lewis acids.
Yes, both BCl3 and SiCl4 are electron deficient. Whereas B atom has a vacant 2p orbital, Si atom at the same time has vacant 3d-orbitals. Both these atoms can take up electron pairs from electron donor species.
Question 10.
Write resonating structures for CO32- and HCO–3.
Answer:
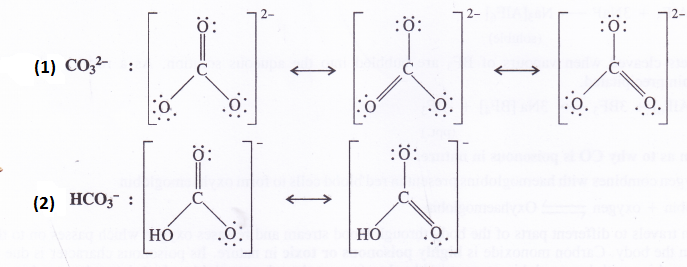
Question 11.
What is the state of hybridisation of carbon in
(a) CO2-
(b) diamond
(c) graphite ?
Answer:
(a) CO2-3 (sp2), (b) Diamond (sp3), (c) Graphite (sp2).
Question 12.
Explain the difference in properties of diamond and graphite on basis of their structures.
Answer:
Diamond
- It generally occurs in the free state.
- It is the hardest substance known.
- Carbon atoms are sp3 hybridised.
- It is a bad conductor of heat and electricity.
- It is transparent with high refractive index (2-42).
- It has very high melting point (4000 K or more).
Graphite
- It occurs in the free state but can also be prepared artificially.
- It is soft and greasy.
- Carbon atoms are sp2 hybridised.
- It is a good conductor of heat and electricity.
- It is opaque.
- It has comparatively low melting point (1800 K
Question 13.
Rationalise the given statements and give chemical reaction :
lead(II) chloride reacts with Cl2 to give PbCl4.
lead(IV) chloride is highly unstable towards heatlead is known not to form an iodide, Pbl4.
Answer:
(a) Lead (Pb) in 4-2 oxidation state i.e., Pb (II) is more stable than in 4- 4 oxidation state i.e., Pb (IV). This means that Pb (II) chloride will not react with chlorine to form Pb (IV) chloride
PbCl2(s) + Cl2(g) → PbCl4(g)
(b) Lead in (II) oxidation state is more stable than in lead in (IV) oxidation state.Therefore, lead (IV) chloride is highly unstable to heat. It decomposes upon heating to form lead (II) chloride.
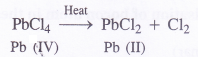
(c) Lead is not known to form Pbl4 because L ion being a powerful reducing agent reduces Pb4+ ion to Pb2+ ion in solution. Thus, Pbl2 is generally formed.
![]()
Question 14.
Suggest reasons why the B-F bond lengths in BF3 (130 pm) and BF–4 (143 pm) differ ?
Answer:
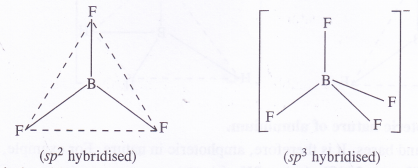
Difference in bond lengths is due to difference in the state of hybridisation of boron in the two fluorides.
Question 15.
If B-Cl bond has a dipole moment, why does BCI3 have zero dipole moment ?
Answer:
B-Cl bond-has a certain dipole moment because it is of polar nature. But BCl3 has zero dipole moment since the molecule is symmetrical (planar) just like BF3 in which bond polarities cancel out.
Question 16..
Aluminium trifluoride is insoluble in anhydrous HF but dissolves on addition of NaF. Aluminium trifluoride precipitates out of the resulting solution when gaseous BF3 is bubbled through it. Give reason.
Answer:
Aluminium trifluoride (AlF3) is insoluble in anhydrous HF because of its covalent nature. However, it forms a complex compound on reacting with NaF which is water soluble.
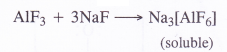
The complex gets cleaved when vapours of BF3 are bubbled into the aqueous solution. As a result, aluminium trifluoride is again precipitated.
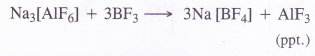
Question 17.
Suggest a reason as to why CO is poisonous in nature.
Answer:
In the lungs, oxygen combines with haemoglobins present in red blood cells to form oxyhaemoglobin
![]()
Oxyhaemoglobin travels to different parts of the body through blood stream and releases oxygen which passes on to the various tissues in the body. Carbon monoxide is highly poisonous or toxic in nature. Its poisonous character is due to its tendency to combine with haemoglobin present in blood to form carboxy haemoglobin which is not in a position to carry the inhaled oxygen to different parts in the body. This will lead to suffocation and ultimately to death.
Haemoglobin + CO —> Carboxyhaemoglobin
We may conclude that carbon monoxide reduces the oxygen carrying capacity of haemoglobin.
Question 18.
How is excess content of CO2 responsible for global warirfing ?
Answer:
We know that CO2 is very much essential for plants to carry photosynthesis. The gas is produced during various types of combustion reactions and is released into the atmosphere. It is taken up by plants as pointed above. Thus, a carbon dioxide cycle works in the atmosphere and its percentage remains nearly constant. However, over the years, combustion reactions have enormously increased. As a result, CO2 gas is now present in excess in the atmosphere. Like methane, it also behaves like a green house gas and absorbs heat radiated by the earth. Some of the heat is released into the atmosphere while the rest is radiated back to earth. This has resulted in global warming over the years and has brought about major climatic changes.
Question 19.
Explain the structure of diborane and boric acid.
Answer:
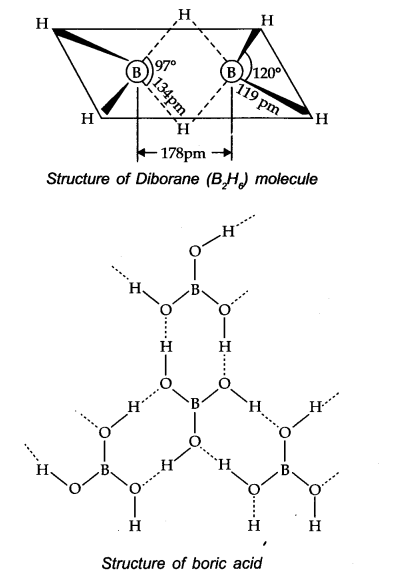
Question 20.
What happens when
(a) Borax is heated strongly
(b) Boric acid is added to water
(c) Aluminium is treated with dilute NaOH
(d) BF3 is reacted with ammonia ?
Answer:
(a) When powdered borax is heated strongly in the flame of bunsen burner, it forms a colourless transparent glassy (glass-like) bead made of sodium meta borate and boric anhydride.
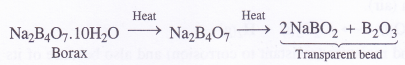
(b) It dissolves in water as it is electron deficient in nature.
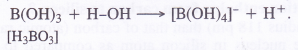
(c) Aluminium dissolves in NaOH solution to form a soluble complex, liberating hydrogen gas.
![]()
(d) BF3 (Lewis acid in nature) forms an addition compound with NH3 (Lewis base in nature).
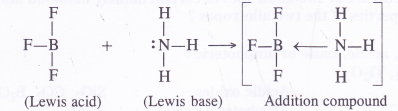
Question 21.
Explain the following reactions :
(a) Silicon is heated with methyl chloride at high temperature in the presence of copper
(b) Silicon dioxide is treated with hydrogen fluoride
(c) CO is heated with ZnO
(d) Hydrated alumina is treated with aqueous NaOH solution.
Answer:
(a) Silicon upon heating with methyl chloride to about 300°C in the presence of copper catalyst reacts as follows :
![]()
The compound upon hydrolysis leads to the formation of silicon polymers.
(b) On reacting silicon dioxide with hydrogen fluoride, silicon tetrafluoride (SiF4) is formed.
SiO2 + 4HF → SiF4 + 2H2O
SiF4 reacts further with hydrogen fluoride to form hydrofluorosilicic acid.
SiF4 + 2HF → H2SiF6
(c) ZnO is reduced to Zn by CO which is a strong reducing agent.
![]()
(d) The two compounds react upon heating under pressure to form a soluble complex.
Al2O3(s) + 2NaOH(aq) + 3H2O(l) —> 2Na[Al(OH)4](aq)
Question 22.
Give reasons :
(1) Con. HNO3 can be transported in aluminium coptainer.
(2) Graphite is used as lubricant.
(3) Diamond is used as an abrasive.
(4) Aluminium alloys are used to make aircraft body.
(5) Aluminium utensils should not be kept in water overnight.
(6) Aluminium wire is used to make transmission cables.
Answer:
- Cone. HNO3 initially reacts with aluminium to form aluminium oxide (Al2O3) which forms a protective coating inside the container. The metal becomes passive and does not react with the acid any more. Therefore, the acid can be safely stored in aluminium container.
- Graphite is used as lubricant because of its soft and greesy nature. This is probably due to the presence of layers in the arrangement of carbon atoms in graphite.
- Diamond is used as an abrasive because of its extremely hard nature (hardest substance known). Actually, the carbon atoms in diamond are very closely packed. This is responsible for the hardness of diamond.
- Alloys of aluminium Magnalium and Duralumin both of which contain about 95 % of the metal are used for making aircraft body. Actually, both of them are light, tough and quite resistant to corrosion. Therefore, they are used in making bodies of air crafts.
- Although the metal as such is not affected by water, but when kept overnight, it may be slowly affected by moisture in the presence of oxygen (air).
2Al (s) + O2(g) + H2O(l) → Al2O3(s) + U2(g) - The metal is not affected by air and moisture (resistant to corrosion) and also because of its good conductivity, it is used to make transmission cables.
Question 23.
Explain why is there a phenomenal decrease in ionization enthalpy from carbon to silicon ?
Answer:
This is because of large atomic size of silicon (atomic radius 118 pm) than that of carbon (atomic radius = 77 pm). As a result, the electrons are less closely attracted to the nucleus in silicon atom as compared to carbon atom. The ionization enthalpy of silicon is, therefore, less. For example, A,H] of silicon is
786 kJ mol-1 .
Question 24.
How would you explain the lower atomic radius of Ga as compared to Al ?
Answer:
The atomic radius of gallium is less than that of aluminium. Down the group, the atomic radius is expected to increase. The decrease in atomic radius of gallium (135 pm) as compared to that of aluminium (143 pm) may be attributed to the presence of ten elements of the first transition series (Z = 21 to 30) which have electrons in the 3d Since d-orbitals have large size than the p-orbitals, the intervening electrons do not have sufficient shielding effect to counter the increase in the nuclear charge. Therefore, the effective nuclear charge in case of Ga (Z=31) is more than the expected value. This decreases its atomic radius which otherwise is expected to increase. The ionic radii of these elements increase regularly as is evident from their values.
Question 25.
What are allotropes ? Sketch the structure of two allotropes of carbon namely diamond and graphite. What is the impact of structure on physical properties of the two allotropes ?
Answer:
Elemental carbon exists in a number of allotropic forms which are both crystalline and amorphous in nature. A few important out of these are briefly discussed. Carbon exists in two types of allotropic forms. These are crystalline and amorphous.
Crystalline Allotropic Forms
Diamond and graphite are the two crystalline forms but they differ in the arrangement of the carbon atoms and hence have different physical properties.
Diamond
Diamond is the hardest crystalline form of carbon and is present in different parts of world as a constituent of hard rocks. Diamonds are mainly found in South Africa, Brazil, Australia, British Guiana etc. In India, diamonds are mainly found in Golconda and Panna. In nature, diamonds occur as transparent octahedral crystals with curved surfaces. Their beauty is revealed only when these are properly cut and polished.
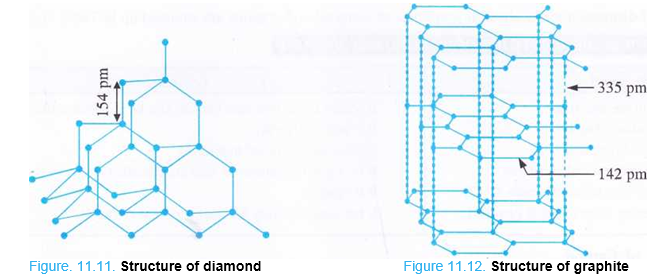
Structure of Diamond. In diamond, all the carbon atoms are sp3 hybridised and are arranged tetrahedrally in space. Each carbon atom is linked to three other carbon atoms by covalent bonds. This results in a three dimensional network as shown in the Fig. 11.11.1 Each C—C bond length is 154 pm. As the carbon atoms in diamond are very closely packed in space, it is, therefore, very hard with density equal to 3-5 g cm3 and has a very high melting point (4000 K or even more). Because of high refractive index (2-42), it produces maximum total internal reflection responsible for its bluish green colour. Since all the four valence electrons pf the carbon atom are involved in bond formation, in the absence of free electrons, diamond is bad conductor of electricity. It is also a poor conductor of heat.
Uses of Diamond.
- Due to its hardness, diamond is used for cutting marble, granite and glass.
- It is used as an abrasive and for polishing hard surfaces.
- It is used in making special surgical knives.
- Dies made from diamond are used for drawing wires from the metals.
- Diamonds when properly cut and polished are used as precious stones or gems.
Question 26.
Classify fallowing oxides as neutral, acidic, basic or amphoteric :
CO, B2O3,SiO2, CO2, Al2O3, PbO2, Tl2O3.
Answer:

Question 27.
In some of the reactions, thallium resembles aluminium, whereas in others, it resembles with group I metals. Support this statement by giving some evidences.
Answer:
Aluminium (Al) generally exhibits + 3 oxidation state in its compounds. Thallium (Tl), the last member of the group-13, is expected to show oxidation states of + 3 and +1 (due to inert pair effect). Thus, the metal resembles aluminium in exhibiting + 3 oxidation state. Both form trichlorides TlCl3 and AlCl3 At the same time, it also resembles alkali metals of group 1 in exhibiting + 1 oxidation state (e.g., both thallium and sodium form monochlorides TlCl and NaCl).
Tl shows both the oxidation state +1 and +3 due to inert pair effect. Tl forms basic oxide like group I elements. TlO2 is strongly basic.
Question 28.
When metal X is treated with sodium hydroxide, a white precipitate (A) is obtained, which is soluble in excess of NaOH to give soluble complex (B). Compound (A) is soluble in dilute HCl to form compound (C). The compound (A) when heated strongly gives (D), which is used to extract the metal. Identify (X), (A), (B), (C) and (D). Write suitable equations to support their identiti(P.I.S.A. Based)
Answer:
The data suggests that the metal ‘X’ is aluminium. The reactions in which aluminium participates leading to the ‘ formation of compounds (A), (B), (C) and (D) are given :
(1) Aluminium (X) reacts with NaOH upon heating to form a white precipitate of Al(OH)3 e., compound (A) which dissolves in excess of NaOH to form soluble complex ‘B’
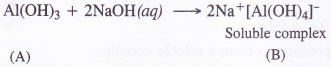
(2) The compound (A) dissolves in dil HC1 to form aluminium chloride (C)
![]()
(3) Upon heating Al(OH)3 is converted into alumina (D)
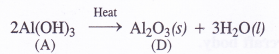
Al2O3 is used in the extraction of the Al metal.
Question 29.
What do you understand by (a) inert pair effect (b) allotropy (c) catenation ?
Answer:
(a) Inert pair effect: The pair of electron in the valence shell does not take part in bond formation is called inert pair effect.
(b)Allotropy: It is the property of the element by which an element can exist in two or more forms which have same chemical properties but different physical properties due to their structures.
(c)Catenation: The property to form chains or rings not only with single bonds but also with multiple bonds with itself is called catenation.
For example, carbon forms chains with (C-C) single bonds and also with multiple bonds (C = C or C = C).
Question 30.
A certain salt X gives the following results :
(1) Its aqueous solution is alkaline to litmus.
(2) It swells up to a glassy material Y on strong heating.
(3) When cone. H2SO4 is added to a hot solution of X, white crystals of an acid Z separate out.
Write equations for all the above reactions and identify X, Y and Z.
Answer:
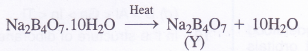
The data suggests that the salt ‘X’ is Borax (Na2B4O7.10H2O)
(1) The aqueous solution of ‘borax is of basic nature and turns red litmus blue.
![]()
(2) Borax swells in size upon strong heating and loses molecules of water of crystallisation to form
solid (Y)
(3) Upon reacting with cone. H2SO4, borax forms boric acid (H3BO3). When crystallised from the solution, it is in the form of white crystals (Z)
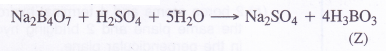
Question 31.
Write balanced equations for the following :
(1) BF3 + LiH→
(2) B2H6 + H2O—>
(3)NaH + B2H6—>
(4) H3BO3—>
(5) Al + NaOH—>
(6) B2H6 + NH3→
Answer:
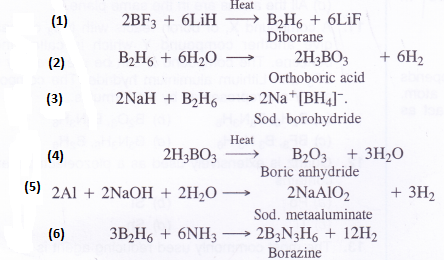
Question 32.
Give one method for industrial preparation and one for laboratory preparation of CO and CO2
Answer:
Carbon Monoxide (CO)
Preparation of carbon monoxide
- Carbon monoxide is a constituent of water gas also called synthesis gas (CO + H2) and is formed by passing steam over red hot coke.
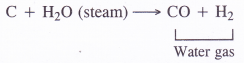
- Carbon monoxide can be separated by liquefaction.If air is passed over red hot coke instead of’steam producer gas is formed which is a mixture of CO and N2 From producer gas, CO can be removed by liquefaction
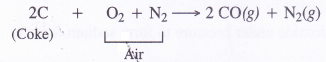
- Carbon monoxide can be,prepared by passing carbon dioxide through red hot charcoal.
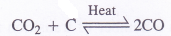
From the mixture, CO2 can be removed by passing the mixture into water under pressure. - Oxides of certain metals upon heating with powdered coke are reduced to carbon monoxide

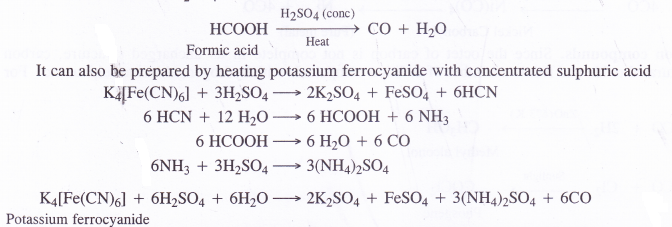
- Laboratory preparation. In the laboratory carbon monoxide can be prepared by the dehydration of formic acid with concentrated H2SO4
Carbon Dioxide (CO2)
Preparation of Carbon dioxide
- Carbon dioxide can be prepared by the following methods.
C + O2 →CO2
CH4 + 2O2 → CO2 + 2H2O - It can also be obtained by the thermal decomposition of certain carbonates of non-alkali metals.
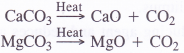
- It can be prepared by the action of dilute acids on certain carbonates and bicarbonates of metals.
Na2CO3 + 2HCl (dil.) → 2NaCl + H2O + CO2
NaHCO3 + H2SO4(dil.)→ NaHS04 + H2O + CO2
- In the laboratory, carbon dioxide is prepared by the action of dilute hydrochloric acid on calcium carbonate.
CaCO3 + 2HCl (dil)→ CaCl2 + H2O + CO2
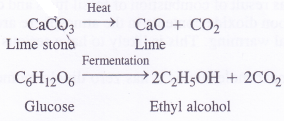
- On commerical scale, carbon dioxide is obtained as a by-product in the decomposition of lime stone to form lime and in the manufacture of ethyl alcohol as a result of fermentation of glucose (present in cane sugar).
Question 33.
An aqueous solution of borax is :
(a)neutral
(b) amphoteric
(c) basic
(d) acide
Answer:
(c) is the correct answer.
Question 34.
Boric acid is polymeric due to :
(a) its acidic nature
(b) the presence of hydrogen bonds
(c) its monobasic nature
(d) its geometry.
Answer:
(b) The acid is polymeric due to hydrogen bonding.
Question 35.
The type of hybridisation of boron in diborane is :
(a) sp
(b) sp1
(c) sp3
(d) dsp2
Answer:
(c) Boron is sp3 hybridised in diborane.
Question 36.
Thermodynamically the most stable form of carbon is
(a) diamond
(b) graphite
(c) fullerenes
(d) Coal
Answer:
(b) graphite is thermally the most stable form.
Question 37.
Elements of group 14 :
(a) exhibit oxidation state of +4 only
(b) exhibit oxidation state of +2 and +4
(c) form M2‘ and M4+ ions
(d) form M2+ and M4+
Answer:
both (b) and (d) are correct answers. The variable oxidation states are due to inert pair effect.
Question 38.
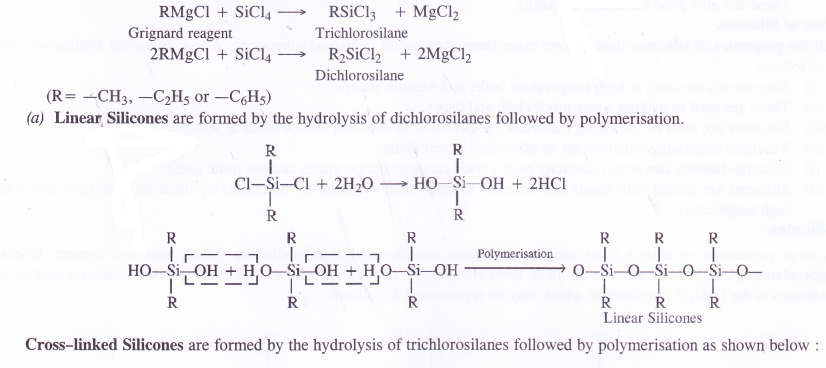
If the starting material for the manufacture of silicones is RSiCl3, write the structure of the product formed.
Answer:
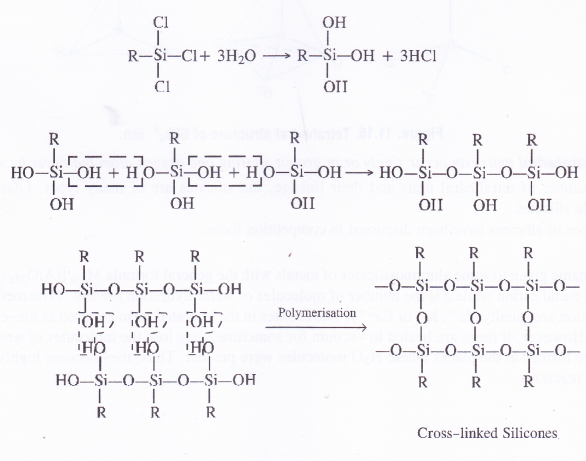
Silicones
Silicones are the synthetic organosilicone polymers containing Si—O—Si linkages. These are represented by the general formula (R2SiO)„ in which R may be alkyl (methyl or ethyl or phenyl groups). Since the general empirical formula is similar to a ketone (R2CO), the name silicone has been given to these polymers.
Structure of Silicones
Silicones are of two types. These may be either linear polymers or cross-linked in nature. Both of them are formed by the action of SiCl4 on Grignard reagents followed by hydrolysis leading to polymerisation.
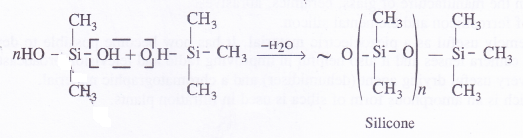
NCERT Solutions for Class 11 Chemistry Chapter 11(A) Some p-Block Elements (Group 15 Elements)
These Solutions are part of NCERT Solutions for Class 11 Chemistry. Here we have given NCERT Solutions for Class 11 Chemistry Chapter 11(A) Some p-Block Elements (Group 15 Elements)
Question 1.
Discuss the general characteristics of group 15 elements with reference to their electronic configuration, oxidation state, atomic size, ionisation enthalpy and electronegativity.
Answer:
For answer, consult Section 11.3..
Question 2.
Why is the reactivity of nitrogen different from that of phosphorus ?
Answer:
Molecular nitrogen exists as a diatomic molecule (N2) in which the two nitrogen atoms are linked to each other by triple bond (N=N). It is a gas at room temperature. Multiple bonding is not possible in case of phosphorus due to its large size. It exists as P4 molecule (solid) in which P atoms are linked to
one another by single covalent bonds. Because of greater bond dissociation enthalpy (946 kJ mol-1) of N=N bond, molecular nitrogen is very less reactive as compared to molecular phosphorus.
Question 3.
Discuss the trends in chemical reactivity of group 15 elements.
Answer:
For answer, consult chemical properties of nitrogen family (Section 11.4).
Question 4.
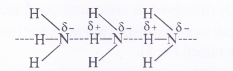
Why does NH3 form hydrogen bonding while PH3 does not ?
Answer:
The N—H bond in ammonia is quite polar on account of the electronegativity difference of N (3-0) and H (2 .1). On the contrary, P—FI bond in phosphine is almost non-polar because both P and H atoms have almost same electronegativity (21). Due to polarity, intermolecular hydrogen bonding is present in the molecules of ammonia but not in those of phosphine.
Question 5.
How is nitrogen prepared in the laboratory ? Write the chemical equations of the reactions involved.
Answer:
For answer, consult Section 7.6.
Question 6.
How is ammonia manufactured industrially ?
Answer:
For answer, consult Section 7.7.
Question 7.
Illustrate how copper metal gives different products on reaction with HNO3.
Answer:
For answer, consult properties of HNO3 (Section 7.8).
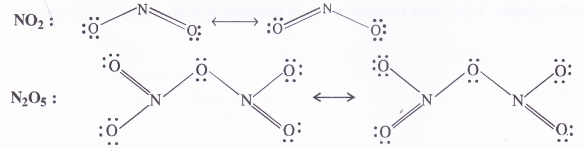
Question 8.
Give the resonating structures of NO2 and N2O5
Answer:
Question 9.
The HNH angle value is higher than those of HPH, HAsH and HSbH angles ; why ?
Answer:
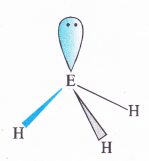
![]()
The central atom (E) in all the hydrides is sp3 hybridised. However, its electronegativity decreases and atomic size increases down the group. As a result, there is a gradual decrease in the force of repulsion in the shared electron pairs around the central atom. This leads to decrease in the bond angle. For more details, consult text part Section 7.4.
Question 10.
Why does R3P=0 exist but R3N=0 does not (R is an alkyl group) ?
Answer:
Nitrogen does not have vacant d-orbitals on its valence’shell. Therefore, it cannot extend its covalency to five and dn-pn bonding is not possible. As a result, the molecules of R3N=O does not exist. However, phosphorus and rest of the members of the group 15 have vacant rf-orbitals in the valence shell which can be involved in dn-pn bonding. Under the circumstances, R3P=O molecule can exist.
Question 11.
Explain why is NH3 basic while PH3 is feebly basic in nature.
Answer:
Both nh3 and ph3 are Lewis bases due to the presence of lone electron pair on the central atom. However, NH3 is more basic than PH3. The atomic size of nitrogen (Atomic radius = 70 pm) is less than that of phosphorus (Atomic radius = 110 pm) As a result, electron density on the nitrogen atom is more than on phosphorus. This means that electron releasing tendency of ammonia is also more and is therefore, a stronger base than phosphine.
Question 12.
Nitrogen exists as diatomic molecule (N2) while phosphorus as tetra-atomic molecule (P4). Why ?
Answer:
For answer, consult Section 7.3 (Physical properties of nitrogen family).
Question 13.
Write the main difference between the properties of white and red phosphorus.
Answer:
For answer, consult Section 7-11.
Question 14.
Why does nitrogen show catenation properties less than phosphorus ?
Answer:
The valence shell electronic configuration of N is 2s22p3. In order to complete the octet, the two nitrogen atoms share three electron pairs in the valence p-sub-shell and get linked by triple bond (N=N). Thus molecular nitrogen exists as discrete diatomic species and there is no scope of any self linking or catenation involving a number of nitrogen atoms. However, in case of phosphorus, multiple bonding is not feasible due to Comparatively large atomic size of the element. For details, consult 7.3. Molecular phosphorus exists as tetra-atomic molecule (P4) in white phosphorus. These tetrahedrons are further linked by covalent bonds to form red variety which is in polymeric form. Thus, catenation in nitrogen is less than in phosphorus.
Question 15.
Give one disproportionation reaction of phosphorus acid (H3PO3).
Answer:
Upon heating to about 573 K, phosphorus acid undergoes disproportionation as follows :

Question 16.
Can PC5 act as oxidising as well as reducing agent ? Justify.
Answer:
In general, the molecules of a substance can behave as a reducing agent if the central atom is in a position to increase its oxidation number. Similarly, they can act as an oxidising agent if the central atom is in a position to decrease its oxidation number. Now, the maximum oxidation state or oxidation number of phosphorus (P) is +5. It cannot increase the same but at the sametime can decrease its oxidation number. In PCl5, oxidation number of P is already +5. It therefore, cannot act as a reducing agent. However it can behave as an oxidising agent in certain reactions in which its oxidation number decreases. For example,
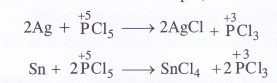
Question 17.
Why are pentahalides more covalent than trihalides in the members of the nitrogen family ?
Answer:
The electronic configuration of the elements of nitrogen family (group 15) is ns2p3. Because of the inert pair effect, the valence 5-electrons cannot be released easily for the bond formation. This means that the elements can form trivalent cation (E3+) by releasing valence p-electrons while it is difficult to form pentavalent cation (E3+). Under the circumstances, if all the five valence electrons are to be involved in the bond formation, the compounds showing pentavalency or +5 oxidation state must be of covalent nature. This is particularly the case in the higher members (Sb, Bi) of the family where the inert pair effect is quite prominent.
Question 18.
Why is BiH3 the strongest reducing agent amongst all the hydrides of group 15 elements ?
Answer:
Down the group, the atomic size of the element (E) increases and the bond length of the corresponding E—H bond also increases. This adversely affects the bond dissociation enthalpy. This means that amongst the trihydrides of the members of nitrogen family, the bond dissociation enthalpy of Bi—H bond is the least. Therefore, BiH3 is the strongest reducing agent among the hydrides of group 15 elements.
Question 19.
Why is N2less reactive at room temperature ?
Answer:
In the nitrogen molecule (N2), two atoms of nitrogen are linked by triple bond (N = N). Due to small atomic size of the element (atomic radius = 70 pm), the bond dissociation enthalpy is very high (946 kJ mol-1). This means that it is quite difficult to cleave or break the triple bond at room temperature. As a result, N2 is less reactive at room temperature.
Question 20.
Mention the conditions required for the maximum yield of ammonia.
Answer:
In Haber’s process, ammonia is formed by the following reaction.
![]()
According to Le-chatelier’s principle, the favourable conditions for the maximum yield of ammonia are :
Low temperature : But optimum temperature of 700 K is necessary to keep the forward reaction in progress.
High pressure : Pressure to the extent of about 200 atm is required.
Catalyst & promoter : Ip order to achieve the early attainment of equilibrium, iron oxide acts as catalyst. Along with that; K2O, Al2O3 or Mo metal may act as the promoter to increase the efficiency of the catalyst.
Question 21.
How does ammonia react with blue solution having Cu2+ ions ?
Answer:
When ammonia gas is passed through blue solution containing Cu2+ ions, ammonium hydroxide formed reacts with Cu2+ ions to give a soluble complex with deep blue colour.

Question 22.
How does ammonia react with blue solution having Cu2+ions ?
Answer:
When ammonia gas is passed through blue solution containing Cu2+ ions, ammonium hydroxide formed reacts with Cu2+ ions to give a soluble complex with deep blue colour.
Question 23.
What is bond angle in PH+4 ion higher than in PH3
Answer:
In both PH3 and PH+4 ion, the phosphorus atom is sp3 hybridised. However, in PH3 the central atom has a pyramidal structure due to the presence of lone electron pair on the phosphorus atom.
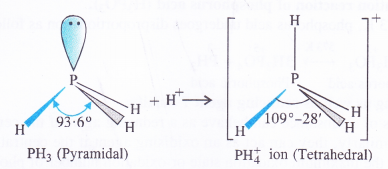
Because of lone pair : shared pair repulsion which is more than that of shared pair : shared pair repulsion, the bond angle in PH3 is nearly 93-6°. In PH+4 ion, there is no lone electron pair on the phosphorus atom. It has a tetrahedralstructure with bond angle of 109°-28′. Thus, the bond angle in PH+4 ion is higher than in PH3.
Question 24.
What happens when white phosphorus is heated with concentrated NaOH solution in an inert atmosphere of CO+4?
Answer:
A mixture of sodium hypophosphite and phosphine gas is formed upon heating the reaction mixture in an inert atmosphere.

Question 25.
What happens when PCl5 is heated ?
Answer:
Upon heating, PCl5 dissociates to give molecules of PCl5 and Cl2. Actually, the two P—Cl (a) bonds with more bond length break away from the molecule leaving three P— C(e) bonds attached to the central P atom since these are more firmly linked
![]()
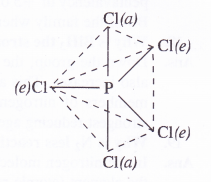
Question 26.
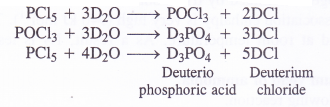
Write the balanced equation for the hydrolytic reaction of PCl5 in heavy water.
Answer:
With heavy water (D2O) ; phosphorus pentachloride (PCl5) reacts as follows :
We hope the NCERT Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements, help you. If you have any query regarding. NCERT Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements, drop a comment below and we will get back to you at the earliest.