NCERT Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry : Some Basic Principles and Techniques
These Solutions are part of NCERT Solutions for Class 11 Chemistry. Here we have given NCERT Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry : Some Basic Principles and Techniques
Question 1.
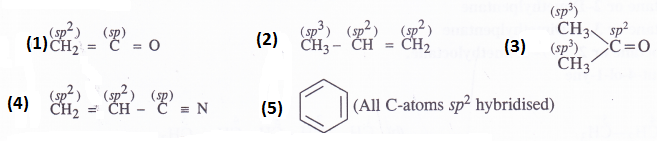
What are the hybridised states of carbon atoms in the following compounds ?
(1) CH2=C=O
(2) CH3CH=CH2
(3) (CH3)2 C=O
(4) CH=CH2 CN
(5) C6H6
Answer:
Question 2.
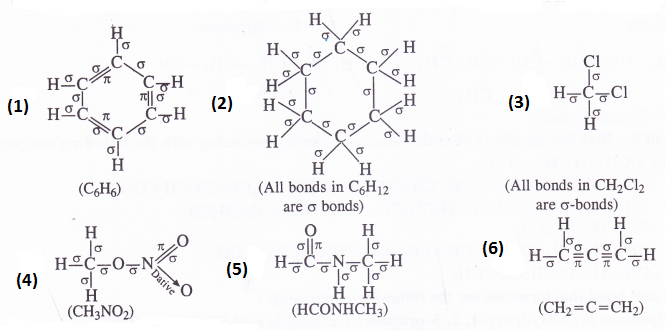
Indicate sigma (σ) and pi (Π) bonds in the following molecules :
(1) C6H6 (2) C6H12 (3) CH2C12 (4) CH3NO2 (5) HCONHCH3 (6) CH2=C=CH2
Answer:
Question 3.
Write bond line formulas for :
(1) Isopropyl alcohol (2) 2, 3-Dimethylbutanal (3) Heptan-4-one
Answer:
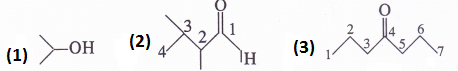
Question 4.
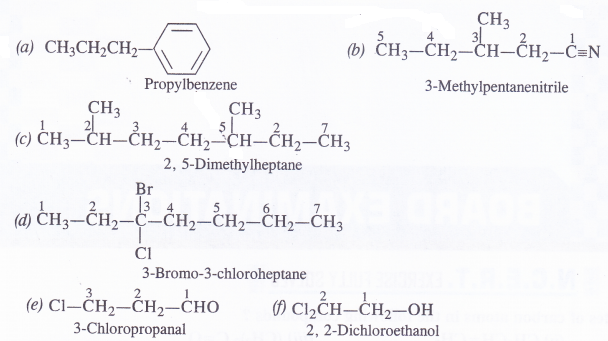
Give the IUPAC name of the following compounds :
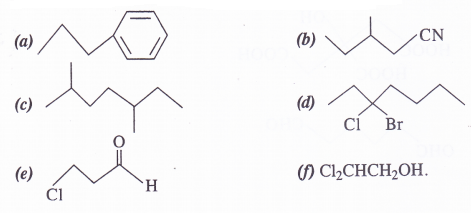
Answer:
Question 5.
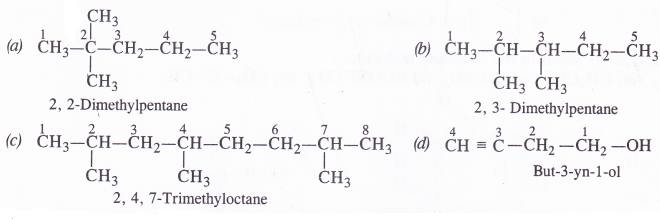
Which of the following represents the correct IUPAC name of the compounds concerned ?
(a) 2, 2-Dimethylpentane or 2-Dimethylpentane
(b) 2, 3-Dimethylpentane or 3, 4-Dimethylpentane
(c) 2, 4, 7-Trimethyloctane or 2, 5, 7-Trimethyloctane.
(d) But-3-yn-l-ol or But-4-ol-l-yne
Answer:
Question 6.
Draw the formulas of the first five members of each homologous series beginning with the following compounds :
(a) HCOOH (b)CH3COCH3 (c) CH2 = CH2
Answer:
(a) HCOOH : CH3COOH, CH3CH2COOH, CH3CH2CH2COOH, CH3CH2CH2CH2COOH
(b) CH3COCH3 : CH3COCH2CH3, CH3COCH2CH2CH3, CH3COCH2CH2CH2CH3 CH3CO(CH2)4CH3.
(c) CH2 = CH2 : CH3CH=CH2, CH3CH2CH=CH2, CH3CH2CH2CH = CH2, CH3CH2CH2CH2CH = ch2
Question 7.
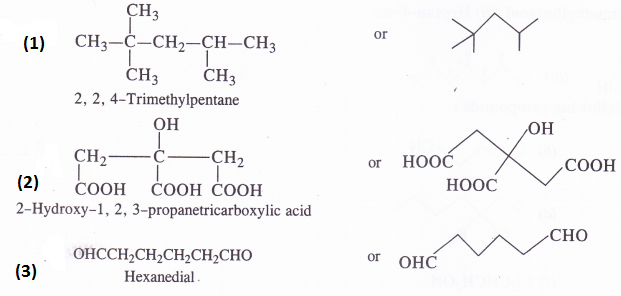
Give the condensed and bond line formulas for the following compounds :
(1) 2,2, 4-Trimethylpentane (2) 2-Hydroxy-l, 2, 3-propanetricarboxylic acid (3) Hexanedial
Answer:
Question 8.
Identify the functional groups in the following compounds :
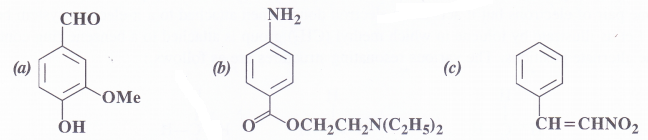
Answer:
- Aldehydic, methoxy, phenolic
- Amino, N-diethylaminopropanoate (Ester)
- Ethylenic double bond, nitro
Question 9.
Which is expected to be more stable : O2NCH2CH2O– or CH3CH2O– and why ?
Answer:
O2NCH2CH2O– is more stable because —NO2 group with -I effect disperses the negative charge on the anion. On the other hand, CH2CH2– group with +1 effect increases the magnitude of the negative charge on the anion and thus destabilises it.

Question 10.
Give the various resonating structures associated with the following molecules :
(1) C6H5OH (2) C6H5NO2 (3) CH3CH=CHCHO (4) C6H5CHO (5) C6H5C H2 (6) CH3CH = CH C H2
Answer:
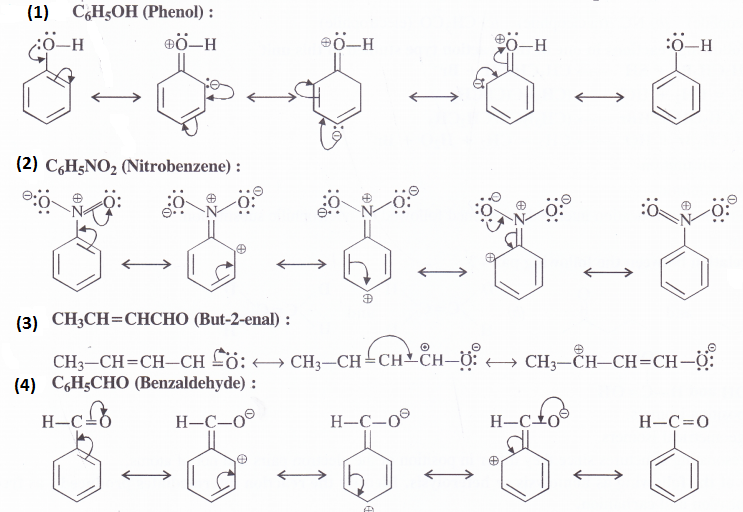
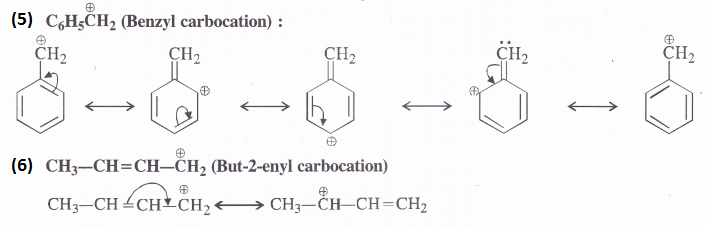
Question 11.
Explain why does an alkyl group act as an electron donor when attached to a Π-electron system.
Answer:
Alkyl group has no lone pair of electrons but it acts as an electron donor when attached to a n-electron system because of hyper conjugation. Let us illustrate by toluene in which methyl (CH3)group is attached to a benzene ring containing three pi-elecrons in the alternate positions. The various resonating structures are as follows :
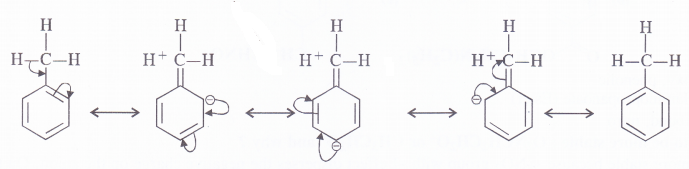
Question 12.
What are nucleophiles and electrophiles. Explain with examples.
Answer:
Electrophiles: The name electrophiles means electron loving. Electrophiles are electron deficient. They may be positive ions or neutral molecules.
Ex: H+, Cl+, Br+, NO2+, R3C+, RN2+, AlCl3, BF3
Nucleophiles: The name nucleophiles means ‘nucleus loving’ and indicates that it attacks the region of low electron density (positive centres) in a substrate molecule. They are electron rich they may be negative ions or neutral molecules.
Ex: Cl– Br–, CN–, OH–, RCR2–, NH3, RNH2, H2O, ROH etc.
Question 13.
Identify the reagents shown in bold in the following equations as nucleophiles or electrophiles.
(a) CH3COOH + OH– → CH3COO– + H2O
(b) CH3COCH3+ NC→ CH3C(CN)OHCH3
(c) C6H6 + CH3CO→ C6H5COCH3
Answer:
(a) OH– (nucleophile) (b) NC– (nucleophile) (c) CH3C+O (electrophile)
Question 14.
Classify the following reactions in one of the reaction type studied in this unit
(1) H3CH2Br + SH– → CH3CH2SH + Br–
(2) (CH3)2C = CH2 + HCl→ (CH3)2C(Cl)CH3
(3) (CH3)3CCH2OH + HBr→ (CH3)2CBr CH2CH3
(4) CH3CH2Br + HO–→ CH2 = CH2 + H2O + Br–
Answer:
(1) Nucleophilic’ substitution
(2) Electrophilic addition
(3) Rearrangement of carbocation intermediate formed followed by nucleophilic substitution.
(4) Elimination.
Question 15.
What is the relation between the following pairs ?
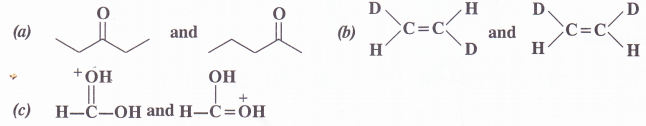
Answer:
These are position isomers.
(1) These are geometrical isomers.
(2) These are resonating structures since they differ in position of the electron pairs and not of atoms.
Question 16.
Classify each of the following as homolysis or heterolysis. Identify the reaction intermediates produced ; as free radical, carbocation and carbanion.
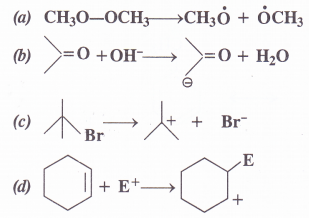
Answer:
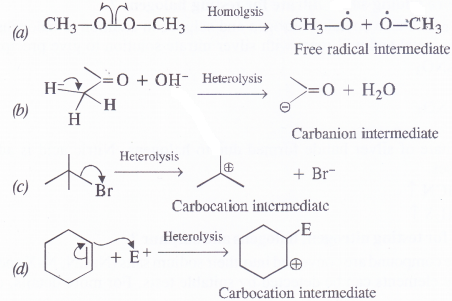
Question 17.
Explain the terms inductive and electromeric effects. Which electron displacement effect explains the following correct order of the acidity of the carboxylic acids ?
(a) CH3COOH > Cl2CHCOOH > ClCH2COOH
(b) CH3CH2COOH > (CH3)2CHCOOH > (CH3)3COOH
Answer:
Electronic displacements in covalent bonds present in the organic molecules may occur either due to the presence of an atom or group of different electronegativity or under the influence of some outside attacking species also called attacking reagent.
The electromeric effect may be defined as :
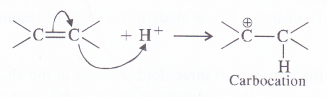
The temporary effect which operates in the organic compounds having multiple bonds i.e. double or triple bonds under the influence of an outside attacking species. As a result, one pi electron pair of the multiple bond gets completely transferred to one of the bonded atoms which is usually more electronegative.
The electromeric effect is shown by a curved arrow ![]() representing the electron transfer originating from the center of the multiple bond and pointing towards one of the atoms which is more electronegative.The effect can be illustrated by the attack of H+ ion on the molecule of alkene.
representing the electron transfer originating from the center of the multiple bond and pointing towards one of the atoms which is more electronegative.The effect can be illustrated by the attack of H+ ion on the molecule of alkene.
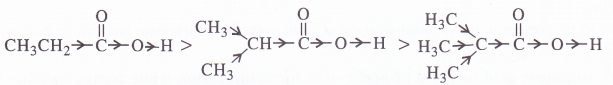
(a) The order of acidity is explained by -I effect of the chlorine atoms. Greater the magnitude of -I effect, easier will be the release of H+ from O-H bond and stronger will be acid.
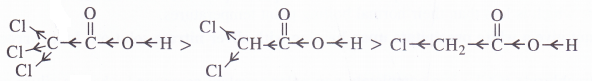
(b) The order of acidity is explained by +I effect of alkyl groups. As the number of alkyl groups increases, the magnitude of +1 effect also increases. As a result, the release of H+ from O-H bond becomes more and more difficult and acidic strength decreases.
Question 18.
Give a brief description of the principle of the following processes taking one example in each case.
(1) Filtration (2) Recrvstallisation (3) Distillation (4) Chromatography
Answer:
(1) Filtration
The hot saturated solution is filtered preferably through a fluted filter paper placed in a glass funnel (Fig. 12.12). The use of the fluted filter paper makes the process of filtration rapid. If the organic compound to be purified has a tendency to crystallise out during filtration, then a hot water funnel is used for filtration (Fig 12.13). The jacket of the hot water funnel is heated from outside and this keeps the solution hot in the glass funnel. This will prevent the formation of crystals during filtration. The insoluble impurities will remain on the filter paper and a clear solution gets collected in the beaker or a dish placed below the funnel.
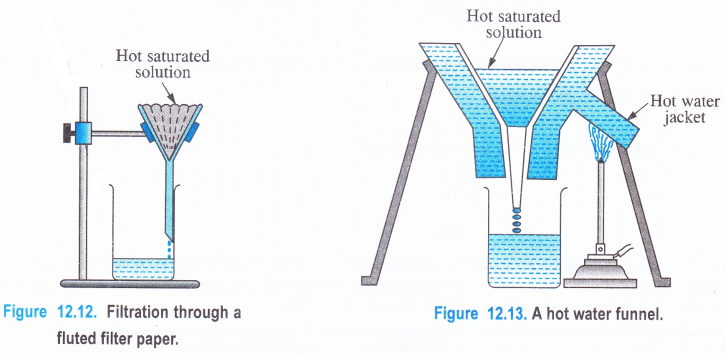
(2) Removal of colour (Recrystallisation). Some times the crystals are coloured due to the presence of some coloured impurities. In such a case, the coloured crystals are redissolved in the same solvent and the saturated solution is prepared. Before filtering the hot solution, a pinch of animal charcoal is added and the solution is boiled for one to two minutes. It is then filtered as before. Thecharcoal adsorbs all the coloured impurities. From the solution, pure crystals can be recovered as described earlier. The process is known as recrystallisation.
(3) Distillation
Distillation is the process of converting a liquid into vapours upon heating and then cooling the vapours back to the liquid state.
The process of simple distillation is used to purify those organic liquids which are quite stable at their boiling points and the impurities present are non-volatile. Liquids such as benzene, toluene, ethanol, acetone, chloroform, carbon tetrachloride can be purified by simple distillation.
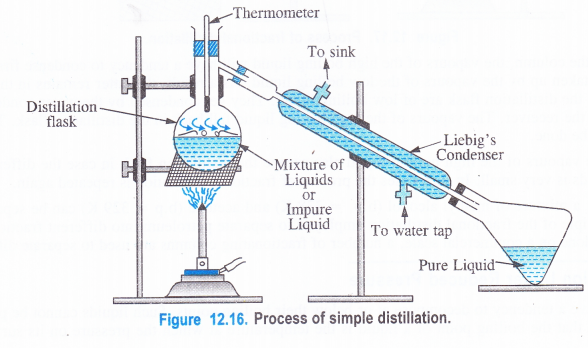
Procedure. The impure liquid is taken in the distillation flask which is fitted with a .condenser called Liebig’s condenser and a thermometer. The flask is heated on a water bath, sand bath or even’directly depending upon the boiling point of the liquid to be distilled. As a result, the liquid changes to its vapours when its boiling point temperature is attained. The vapours then pass through the condenser around which water is circulated as shown in the figure. The vapours condense to form the liquid which is collected in the receiver. The non-volatile impurities are left behind in the distillation flask and can be recovered.
Most of the liquids start bumping when heated. In order to check this, a few pieces of unglazed porcelain or glass beeds are added in the flask.
The process of distillation can also be used to separate a liquid mixture in which the two components present differ in the boiling points ranging from 30 to 50 K e.g., a mixture of diethyl ether (b.p. 308 K) and benzene (b.p. 353 K). When the liquid mixture is heated in the distillation flask, only the vapours of the low boiling liquid will be formed at its boiling point while the high boiling liquid will not change to the vapour state. The vapours of the low boiling liquid will escape and after getting condensed will be collected in the receiver. The high boiling component left in the distillation flask can be recovered from it.
(4) Fractional Distillation
Sometimes, we come across a mixture of two liquids which differ in their boiling point temperatures by 10-20 K. The process of simple distillation will fail here because the vapours of both the liquids will be formed simultaneously and the distillate collected in the receiver will contain both of them. In such cases, the process of fractional distillation is used which employs specially designed columns called fractionating columns. The purpose of the column is to obstruct the movement of the vapours as they rise up. The column actually used depends upon the nature of the liquid mixture.
Procedure. The apparatus used is the same as in the distillation except a fractionating column. Upon heating the vapours of both the liquids will be formed.
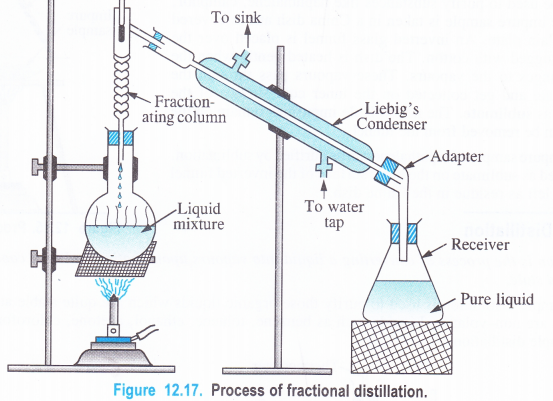
As they rise into the column, the vapours of the high boiling liquid will have a tendency to condense first. In doing so, they release heat which is taken up by the vapours of the low boiling liquid. As a result, the latter remains in the vapour state. The vapours which escape the distillation flask are of low boiling liquid. They get condensed by the water condenser and the liquid formed is collected in the receiver. The vapours of the high boiling liquid fall back in the distillation flask. Thus, the separation of the two liquids can be done.
Sometimes, some vapours of the high boiling liquid also escape the distillation flask in case the difference in the boiling points of the two liquids is very small. In such cases the process of fractional distillation is repeated again.
In the laboratory, a mixture of methyl alcohol (b.p. = 338 K) and acetone (b.p = 329 K) can be separated by fractional distillation. The principle of the fractional distillation employed to separate petroleum into different fractions is also the same but since it has to be done on a commercial scale, a number of fractionating columns are used to separate different fractions.
Question 19.
Describe the method which can be used to separate two compounds with different solubilities in the solvent S.
Answer:
The separation can be done with the help of fractional crystallisation. Choice of the solvent. Inorganic compounds are mostly water soluble. The organic compounds, on the other hand, are generally soluble in organic solvents which may be different for different compounds. In order to make the proper choice of the solvent, the following points must be kept in mind.
- The organic solid must dissolve in the solvent upon heating and must get separated when the hot solution is cooled.
- The impurities should not normally dissolve in the solvent. If at all they dissolve, they should be soluble to such a small extent that they may remain in the mother liquor which gets separated from the crystals.
- The solvent must not react chemically with the organic compound.
The solvents commonly used an of organic nature such as ethyl alcohol, benzene, chloroform, ether, carbon tetrachloride etc. Even water can be used in some cases.
Question 20.
What is the difference between distillation, distillation under reduced pressure and steam distillation ?
Answer:
Distillation is employed in case of volatile liquids associated with non-volatile impurities.
Distillation under reduced pressure is carried to purify liquids which decompose at their boiling point temperatures. Steam distillation is done for the steam volatile liquids associated with water immiscible impurities.
Steam Distillation
This process is used to purify the impure liquid by passing steam and is applicable under the following conditions.
- The liquid must be steam volatile but the impurities present must be non-volatile.
- The liquid must not be miscible with water.
- The liquid must possess sufficiently high vapour pressure at the boiling point temperature of water (373 K).
With the help of steam distillation, the liquids which have boiling points higher than the boiling point of water (373 K) can be distilled at lower temperature under reduced pressure inside the flask. Thus, steam distillation is comparable to distillation under reduced pressure. This can be explained with the help of Dalton’s law of partial pressures as given ahead :
According to the law, the pressure exerted by a gaseous mixture in a container is equal to sum of the partial pressures of the constituent gases provided they do not react chemically, i.e., P = P1 + P2.
Where P = Total pressure of the mixture (atmospheric pressure)
Pi = Vapour pressure of steam
P2 = Vapour pressure of the liquid vapours
It is quite obvious that the vapour pressure of the liquid inside the flask is less than P which is atmospheric pressure. Thus, the liquid boils under reduced pressure and gets distilled along with vapours of steam at a temperature lower than its boiling point temperature.
We can also calculate the ratio of the masses of the organic liquid and water (steam) which escape from the distillation flask as distillate. According to ideal gas equation, PV = nRT. At constant temperature and volume,
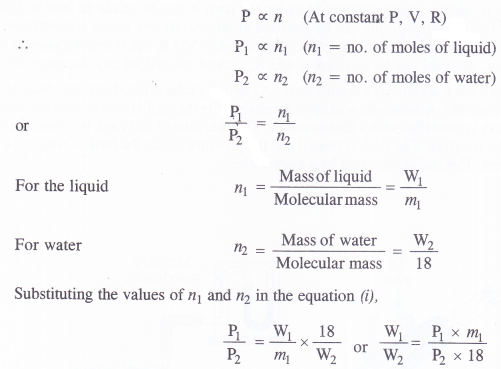
Procedure. The liquid to be purified is mixed with small quantity of water and put in a flask which has two delivery tubes fitted to it, one going into a liquid and the other remaining near the cork. The delivery tube which dips into the liquid is connected to a steam generator and the other one is joined to a Liebig’s condenser which opens into a receiver. The liquid in the flask is heated and steam is bubbled through it. As a result, the liquid changes into vapours while the impurities remain in the flask. The vapours of the liquid along with the steam escape from the flask. These vapours get condensed and are collected in the receiver. The separation of the liquid from water can be done with the help of a separating funnel. The use of a separating funnel has been discussed under differential extraction.
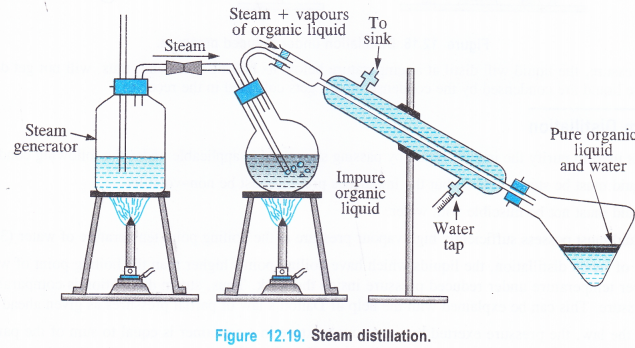
- An impure sample of aniline can be purified by carrying out the steam distillation. The process of steam distillation can also be used to separate a mixture of two organic substances one of which is steam volatile while the other is not. For example, if steam is passed through a mixture of o-nitrophenol and p-nitrophenol taken in the distillation flask, then steam will carry along with it vapours of o-nitrophenol which is low boiling liquid leaving behind p-nitrophenol in the flask which has comparatively high boiling point.
- Fragrance of flowers is due to the presence of some steam. Volatile organic compounds called essential oils present in perfumes, cosmetics etc. These are insoluble in water at room temperature but are miscible in the vapour phase. In other words, these are steam volatile. These can be isolated with the help of steam distillation.
Question 21.
Describe the chemistry of Lassaigne’s test.
Answer:
Lassaigne’s Test,
Nitrogen in an organic compound is detected mainly by Lassaigne ’ s test which is described as follows:
(a) Preparation of Lassaigne’s extract. A small piece of dry sodium metal is heated gently in a fusion tube till it melts to a shining globule. At this stage, a small amount of organic substance is added and the tube is heated strongly for two to three minutes. The red hot tube is plunged into distilled water contained in a china dish. The contents of dish are boiled for couple of minutes, cooled and filtered. The filtrate is known as sodium extract or Lassaigne’s extract.
(b) Test for nitrogen. The Lassaigne’s extract is usually alkaline because excess of sodium reacts with water to form sodium hydroxide. If not, it may be made alkaline by the addition of a few drops of a dilute solution of sodium hydroxide. To a part of the extract, a small amount of a freshly prepared ferrous sulphate solution is added and the contents are warmed. A few drops of ferric chloride solution are then added to the contents and the resulting solution is acidified with dilute hydrochloric acid. The appearance of a bluish green colour due to the formation of ferric ferrocyanide (prussian blue) confirms the presence of nitrogen in the organic compound.
(c) Chemistry of the test. During fusion, carbon and nitrogen present in the organic compound combine with sodium to form sbdium cyanide.

If organic compound contains both nitrogen and sulphur, then they combine with sodium metal to form sodium thiocyanate also called sodium sulphocyanide. This compound will react with ferric chloride to form ferric thiocyanate (or sulphocyanide) which is blood red in colour.

Question 22
Differentiate between the principle of estimation of nitrogen in an organic compound by
(1) Duma’s method
(2) Kjeldahl’s method.
Answer:
In Duma’s method, nitrogen evolved from the organic compound is measured and then estimated. In Kjeldahl’s method, nitrogen present in the organic compound is converted into ammonia which then is estimated volumetrically.
Duma’s Method and Kjeldahl’s Method
Duma’s method can be employed to estimate nitrogen in all organic compounds.
Principle : A known mass of the given organic compound is heated strongly with excess of cupric oxide in an atmosphere of CO2. Carbon and hydrogen are oxidised to CO2 and H2O respectively while nitrogen present in the compound is set free. A small amount of the oxides of nitrogen if formed are reduced back to nitrogen by passing over hot reduced copper gauze.
![]()
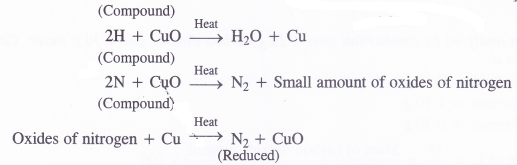
The gaseous mixture is passed through concentrated KOH solution which absorbs both CO2 and H2O vapours. Nitrogen (N2) is not absorbed by KOH and gets collected over it. The volume of nitrogen evolved is noted and from this the amount of nitrogen or its percentage can be calculated by applying suitable calculations.
Apparatus. The apparatus used to estimate nitrogen by Duma’s method is shown in the figure 12.28.
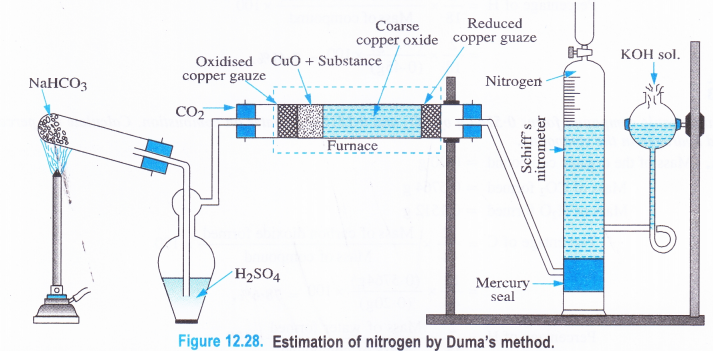
It consists of a combustion tube which is a long hard glass tube open at both ends. It contains in it a roll of oxidised copper gauze to prevent the backward diffusion of the products of combustion. This is followed by small amount of weighed organic compound (about 2 g) mixed with excess of cupric oxide. A layer of coarse cupric oxide covers about half of the combustion tube as shown in the figure. At the end of the tube there is a roll of reduced copper gauze which has been kept to reduce any oxides of nitrogen formed in the oxidation reactions back to nitrogen. In order to collect the evolved nitrogen, the combustion tube is connected to a Schiff’s nitrometer which is a graduated tube containing mercury at its bottom. Mercury acts as a seal and does not allow any liquid to flow back irrthe combustion tube. The nitrometer contains in it about 40 % aqueous KOH solution which absorbs both CO2 and H2O vapours evolved in the combustion reaction. However, nitrogen cannot be absorbed and it get collected over it in the nitrometer. A reservoir attached to the nitrometer helps to record the volume of nitrogen at the atmospheric pressure. The combustion tube is kept in a furnace where it can be heated.
Procedure : The apparatus is fitted as shown in the Figure 12.28. To start with, the tap of the nitrometer is opened and a carbon dioxide formed by heating sodium bicarbonate is passed through the combustion tube in order to expel any air or oxygen present in the tube. After sometime, the tap of nitrometer is closed and the reservoir is raised in order to completely fill the nitrometer tube with KOH solution. The combustion tube is now heated in the furnace. Both CO2 and H2O vapours evolved are absorbed by KOH while nitrogen which is set free gets collected over KOH and its level is, therefore, pushed downwards. Towards the end of the experiment, a strong current of carbon dioxide is passed through the combustion tube to remove last traces of nitrogen if present.
The apparatus is cooled and nitrometer tube is disconnected. The volume of nitrogen is noted after levelling i.e., by keeping the level of KOH in the nitrometer and in the reservoir, same. This will give the volume of nitrogen at the atmospheric pressure which can be recorded from a barometer. The room temperature and the corresponding aqueous tension are also recorded.
Question 23.
Describe the principle of estimation of halogens, sulphur and phosphorus present in an organic compound.
Answer:
All these elements if present in the organic compound, are estimated by Carius Method.
Estimation of Halogens
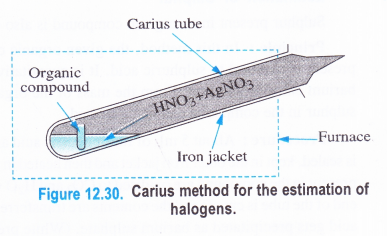
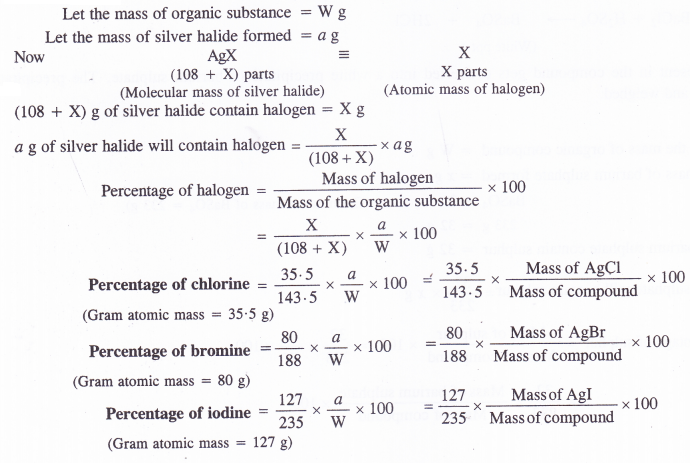
Principle : In carius method, the halogen present in an organic compound is converted into the corresponding silver halide (AgX). From the mass of the organic compound taken and that of silver halide formed, the percentage of halogen in the compound can be calculated. ‘
Procedure: About 5 mL of fuming nitric acid and 0-5 g of silver nitrate are taken in the carius tube made up of hard glass. It is about 50 cm long and is closed at one end. A small amount of the organic compound to be estimated is taken in a small tube which is also placed carefully in the carius tube. The tube is now sealed and is placed in an outer jacket made of iron.
It is heated in a furnace to 550 to 560 K for nearly six hours. Under the reaction conditions, carbon and hydrogen present in the compound are oxidised to CO2 and H2O vapours respectively. Halogen gets converted into silver halide which is precipitated. The high pressure developed inside the tube is released by softening the sealed end with a small flame. A hole gets created through which the gases escape. The end of the tube is then cut off and the contents are transferred into a beaker. The precipitate of silver halide is filtered, washed, dried and is then weighed.
Calulations
Question 24.
Explain the principle of paper chromatography.
Answer:
Principle of Chromatography
The technique of chromatography is based on the difference in the rates at which the components of a mixture are adsorbed on a suitable adsorbent. The material on which the various components are adsorbed is called stationary phase. It is of porous nature and the common substances which can be used in preparing the stationary phase are alumina, silica gel, calcium carbonate or activated charcoal. The mixture to be separated is dissolved in a suitable medium; may be a liquid or a gas. It constitutes the moving phase. The moving phase is made to run on the stationary phase and the separation is based on the principle that the components of the mixture present in moving phase move at different rates through the stationary phase.
Question 25.
Why is nitric acid added to sodium extract before adding silver nitrate for testing halogens ?
Answer:
Problem can arise in case the organic compound which also’contains nitrogen and sulphur in addition to halogens. Both will form NaCN and Na2S on fusion with sodium metal and will react with silver nitrate solution to give precipitates.

These precipitates will interfere’with the precipitate of silver halide formed due to halogens. Nitric acid is added to destroy both these compounds to form volatile gases.
![]()
Question 26.
Why is an organic compound fused with sodium for testing nitrogen, halogens and sulphur ?
Answer:
On fusing with sodium, these elements present in the compound are converted into their sodium salts (NaCN, NaX and Na2S) which are water soluble. From the solution, these elements can be detected by suitable tests.
Test for nitrogen. The Lassaigne’s extract is usually alkaline because excess of sodium reacts with water to form sodium hydroxide. If not, it may be made alkaline by the addition of a few drops of a dilute solution of sodium hydroxide. To a part of the extract, a small amount of a freshly prepared ferrous sulphate solution is added and the contents are warmed. A few drops of ferric chloride solution are then added to the contents and the resulting solution is acidified with dilute hydrochloric acid. The appearance of a bluish green colour due to the formation of ferric ferrocyanide (prussian blue) confirms the presence of nitrogen in the organic compound.
Question 27.
Name a suitable technique of separation of the components from a mixture of calcium sulphate and camphor.
Answer:
The separation can be done by the process of sublimation. Camphor being volatile in nature will undergo sublimation. Calcium sulphate will remain as the residue as it is non-volatile in nature.
Sublimation
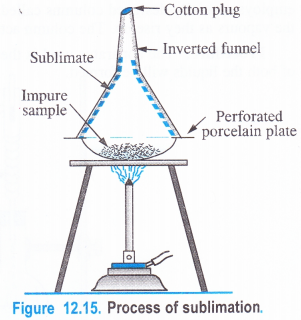
The process of sublimation is applicable to purify those solids which sublime i.e., they directly pass to the vapour state upon heating without passing through the liquid state and the vapours upon cooling give back the solid again. But the impurities associated with them are non-volatile.
The process can be used to purify substances like naphthalene, camphor, benzoic acid, etc. The impure sample is taken in a China dish and is covered by a perforated porcelain plate. An inverted glass funnel is placed over the dish and its stem‘is plugged with cotton. The dish is heated gently when the volatile substance changes to the vapours. These vapours pass through the perforations of the plate and get collected on the inner cold surface of the funnel. This is known as sublimate. The non-volatile impurities remain on the dish. The sublimate can be removed from the funnel.
For example, an impure sample of naphthalene can be purified by sublimation.
Naphthalene is collected as sublimate on the inner surface of the inverted funnel and the impurities are left as residue in the China dish.
Question 28.
An organic liquid vaporises at a temperature below its boiling point in steam distillation. Assign reason.
Answer:
Steam distillation is actually distillation under reduced pressure. The vapour pressures of both water vapours and organic liquid placed in the distillation flask become equal to the atmospheric pressure. This means that both of them will vaporise at a temperature which is less than their normal boiling point temperatures.
Question 29.
Carbon tetrachloride does not give a white precipitate upon heating with silver nitrate solution. Is it correct ?
Answer:
Yes it is correct. Carbon tetrachloride (CCI4) is a completely non-polar covalent compound whereas silver nitrate is ionic in nature. Therefore, they are not expected to react and a white precipitate of silver chloride will not be formed.
Question 30.
A solution of potassium hydroxide is used to absorb carbon dioxide evolved during the estimation of carbon in an organic compound. Explain.
Answer:
Carbon dioxide reacts with KOH present in the solution to form soluble potassium carbonate and can be estimated.
2KOH + CO2→K2CO3 + H2O
Question 31.
It is not advisable to use sulphuric acid in place of acetic acid for acidification while testing sulphur by lead acetate test. Assign reason.
Answer:
Lead acetate will react with sulphuric acid to give white precipitate of lead sulphate. This will interfere with the detection of the test for sulphur.
![]()
Acetic acid (CH3COOH) will not interfere in the detection of sulphur.
Question 32.
An organic compound contains 69% carbon and 4-8% hydrogen, the remainder being oxygen. Calculate the masses of carbon dioxide and water produced when 0-20 g of this compound is subjected to complete combustion.
Answer:
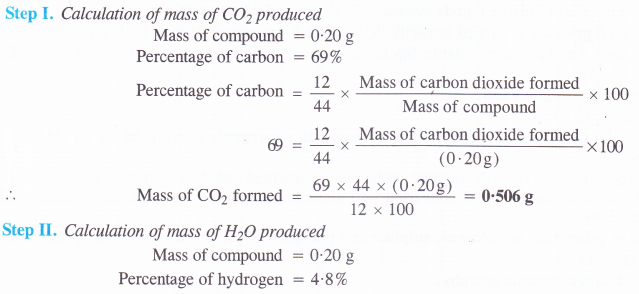
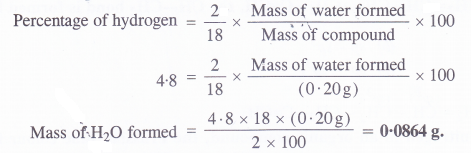
Question 33.
0.50 g of an organic compound was kjeldahlished. The ammonia evolved was passed in 50 cm3 of in H2SO4. The residual acid required 60 cm3 of N/2 NaOH solution. Calculate the percentage of nitrogen in the compound.
Answer:
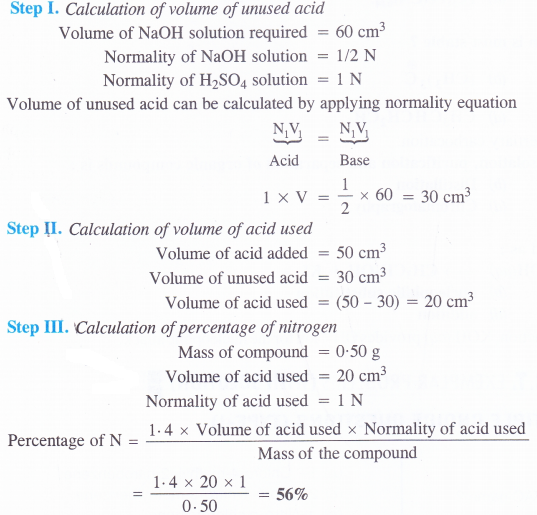
Question 34.
O.3780 g of an organic compound gave 0.5740 g of silver chloride in Carius estimation. Calculate the percentage of chlorine in the compound.
Answer:
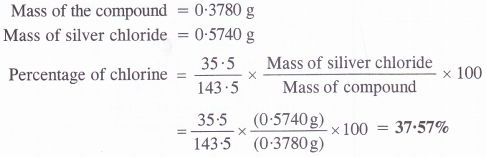
Question 35.
In an estimation of sulphur by Carius method, 0.468 of an organic sulphur compound gave 0.668 g of barium sulphate. Find the percentage of sulphur in the compound.
Answer:

Question 36.
In the organic compound CH2 = CH—CH2—CH2—OCH, the CH—CH2 bond is formed by the interaction of a pair of hybridised orbitals :
(a) sp – sp2
(b) sp – sp3
(c) sp2 – sp3
(d) sp3 – sp3.
Answer:
![]()
Question 37.
In the Lassaigne’s test for nitrogen in an organic compound, the Prussian blue colour is obtained due to the formation of:
(a) Na4[Fe(CN)6]
(b) Fe4[Fe(CN)6]3
(c) Fe2[Fe(CN)6]
(d) Fe3[Fe(CN)6]4.
Answer:
(b) is the correct answer.
Question 38.
Which of the following carbocation is most stable ?

Answer:
(b) is the most stable since it is a tertiary carbocation.
Question 39.
The best and latest technique for isolation, purification and separation of organic compounds is :
(a) Crystallisation
(b) Distillation
(c) Sublimation
(d) Chromatography
Answer:
(d) is the correct answer.
Question 40.
The following reaction is classified as :
CH3CH2I + KOH(aq) → CH3CH2OH + KI
(a) electrophilic substitution
(b) nucleophilic substitution
(c) elimination
(d) addition
Answer:
(b) It is a nucleophile substitution reaction. KOH (aq) provides OH– ion for the nucleophile attack.
We hope the NCERT Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry : Some Basic Principles and Techniques, help you. If you have any query regarding NCERT Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry : Some Basic Principles and Techniques, drop a comment below and we will get back to you at the earliest.