NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
These Solutions are part of NCERT Solutions for Class 11 Chemistry. Here we have given NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons.
Question 1.
How will you account for the formation of ethane during chlorination of methane ?
Answer:
The chlorination of methane proceeds by free radical mechanism. The methyl free radicals (CH3) are converted to ethane during chain termination step.
H3\(\dot { C } \) + \(\dot { C } \)H3 → CH3—CH3
Chlorination. In the chlorination of methane, all the four hydrogen atoms present in the molecule get replaced by one by to form a mixture of different substituted products.
Question 2.
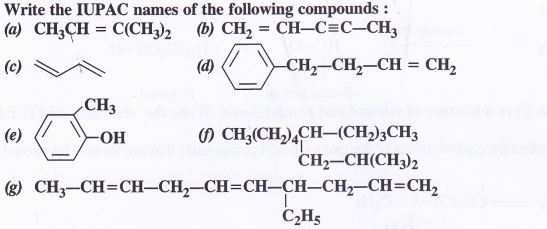
Answer:
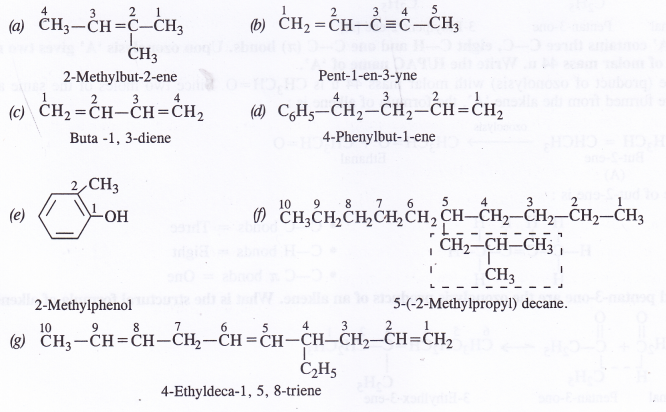
Question 3.

Answer:
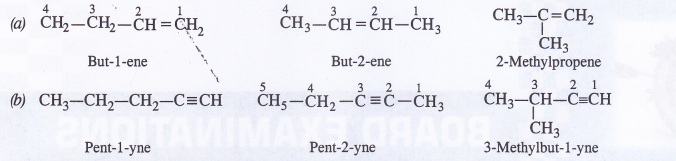
Question 4.
Write the IUPAC names of the products obtained by the ozonolysis of the following compounds :
(i) Pent-2-ene
(ii)3, 4-dimethylhept-3-ene
(iii) 2-Ethylbut-l-ene
(iv) 1-Phenylbut-l-ene.
Answer:
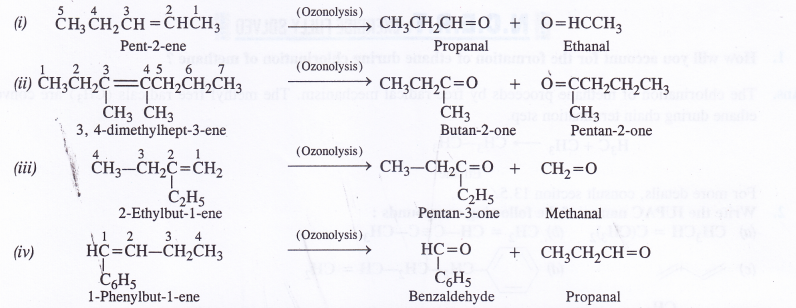
Question 5.
An alkene ‘A’ upon ozonolysis gives a mixture of ethanal and pentan-3-one. Write the structure and IUPAC name of ‘A’.
Answer:
The double bond is present between the carbon atoms of the two carbonyl compounds that are formed by ozonolysis.
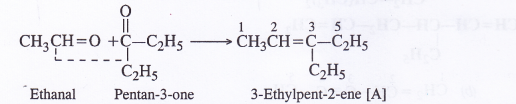
Question 6.
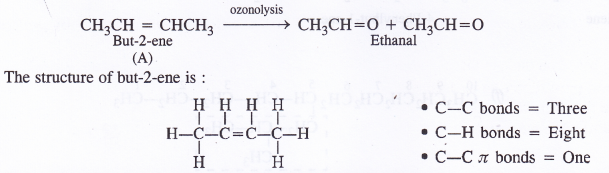
An alkene ‘A’ contains three C—C, eight C—H and one C—C (JI) bonds. Upon ozonolysis ‘A’ gives two moles of an aldehyde of molar mass 44 u. Write the IUPAC name of ‘A’.
Answer:
The aldehyde (product of ozonolysis) with molar mass 44 u is CH3CH=0. Since two moles of the same aldehyde (propanal) are formed from the alkene ‘A’, the formula of alkene is :
Question 7.
Propanal and pentan-3-one are the ozonolysis products of an alkene. What is the structural formula of alkene ?
Answer:
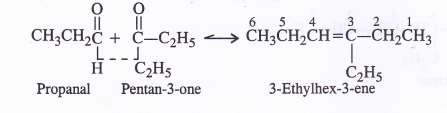
Question 8.
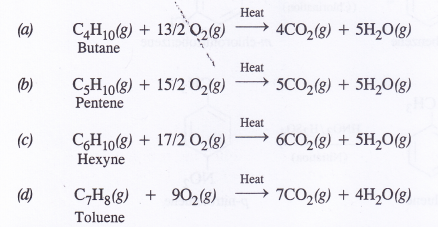
Write the chemical equations for the combustion of the following hydrocarbons :
(a) Butane
(b) Pentene
(c) Hexyne
(d) Toluene.
Answer:
By definition, combustion is for one molecule (one mole) of the substance. The combustion equations may be written as :
Question 9.
Draw cis and trans structures of hex-2-ene. Which isomer will have higher boiling point and why ?
Answer:
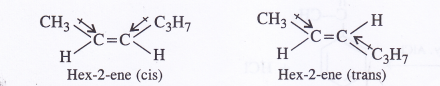
Cis isopier will have higher boiling point because of greater magnitude of dipole-dipole intractions as compared to the trans isomer.
Question 10.
Why is benzene extra-ordinary stable though it contains three double bonds ?
Answer:
It is on account of resonance shown by benzene. Moreover, there is delocalisation of π-electron charge in benzene.
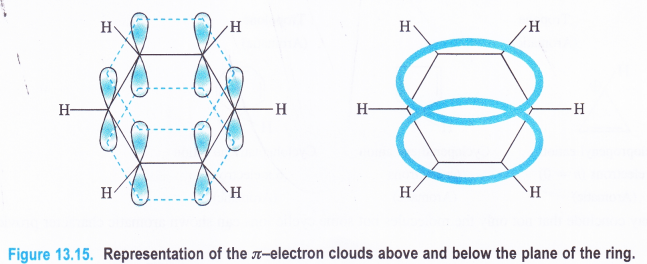
Question 11.
What are tjhe necessary conditions for a system to be aromatic ?
Answer:
We have stated in our earlier discussion that benzene and arenes are aromatic in nature. In general, if a compound is to be aromatic, it must fulfil the following conditions : ,
(i) The compound must be cyclic in nature with atleast one or more double bonds in the ring.
(ii) Contrary to unsaturation as suggested by the molecular formula, it must behave like saturated compounds i.e., must resist addition and take part in the electrophilic substitution reactions.
(iii) The compound must be capable of exhibiting resonance.
(iv) The most essential criteria for the aromatic character is that the compound must obey Huckel’s rule. According the rule, a cyclic compound will behave as aromatic compound if it contains (4n + 2) π electrons, where n may be 1, 2, 3, …. etc.
Question 12.
Explain why the following systems are not aromatic ?
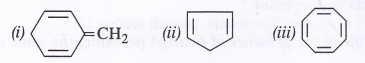
Answer:

Question 13.
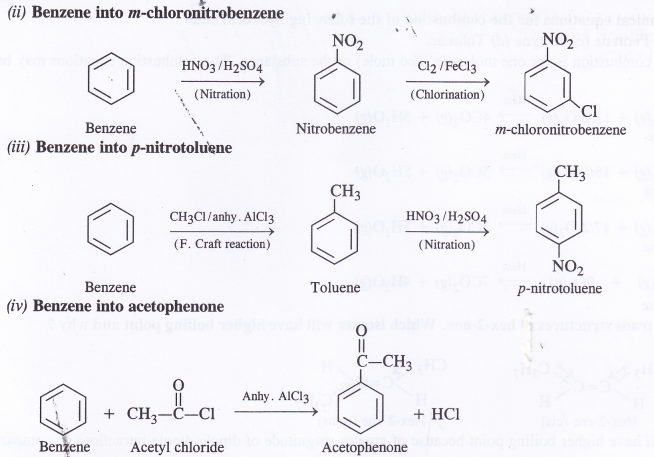
How will you convert benzene into
(i) p-chloronitrobenzene
(ii) m-chloronitrobenzene
(iii) p-nitrotoluene
(iv) Acetophenone ?
Answer:
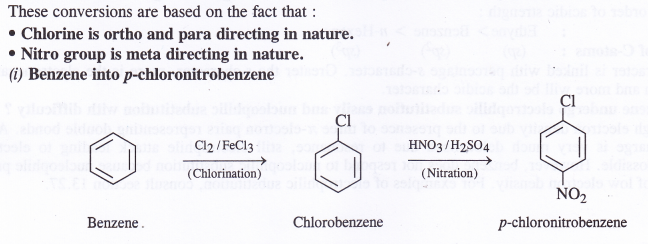
Question 14.
Inthealkane, CH3—CH2—C(CH3)2—CH2—CH2(CH3)2,identify 1°,2°,3° and4° carbon atoms.
Answer:
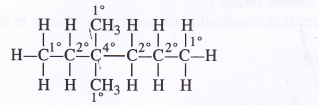
Question 15.
What is the effect of branching of alkane chain on the its boiling point ?
Answer:
Branching of carbon atom chain decreases the boiling point of alkane.
Boiling points. Alkanes are non-polar molecules and the only attractive forces in their molecules are weak van der Waals’ forces. Therefore, the members of the family are low boiling in nature. The addition of each carbon atom (or CH2 group) in the chain increases the boiling point nearly by 30 K. The boiling points of some normal alkanes are given below :

Question 16.
Addition of HBr to propene yields 2-bromopropane whde in the presence of benzoyl peroxide, the same reaction yields 1-bromopropane. Explain and give mechanism.
Answer:
Mechanism of reaction. The addition of HBr in the presence of organic peroxide follows free radical mechanism.
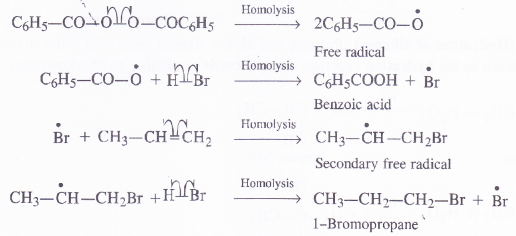
Question 17.
Write the products of ozonolysis of 1, 2-dimethylbenzene (o-xylene). How does the result support Kekule structure of benzene ?
Answer:
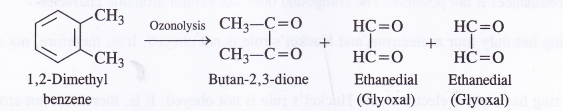
All these products can be possible only in case, there are three double bonds in the ring in the alternate positions. The products of ozonolysis support Kekule structure.
Question 18.
Arrange benzene, n-hexane and ethyne in decreasing order of acidic strength. Also give reason for this behaviour.
Answer:

The acidic character is linked with percentage s-character. Greater the ^-character, more is the electronegativity of the carbon atom and more will be the acidic character.
Question 19.
Why does benzene undergo electrophilic substitution easily and nucleophilic substitution with difficulty ?
Answer:
Benzene has high electron density due to the presence of three ^-electron pairs representing double bonds. Although the electron charge is very much delocalised due to resonance, still electrophile attack leading to electrophilic substitution is possible. However, benzene does not respond to nucleophilic substitution because nucleophile prefers to attack a centre of low electron density.
Role of catalyst in Electrophilic Substitution Reactions.
In the monosubstitution reactions of benzene discussed earlier in the properties of arenes, we have seen that a catalyst is always present which may be either a Lewis acid (Ferric salt or Anhydrous aluminium chloride) or a proton acid (sulphuric acid).
The catalyst is needed to help in generating the electrophile (E+) from the attacking molecule. In fact, the ^-electrons in the benzene are delocalised and are not in a position to cause the polarisation of the attacking molecule.
The catalyst helps in its polarisation which may be illustrated by the chlorination of benzene. The catalyst FeCl3 is a Lewis acid and causes the heterolysis of the chlorine molecule.

Question 20.
How will you convert following into benzene ?
(i) Ethyne
(ii) Ethene
(iii) Hexane
Answer:
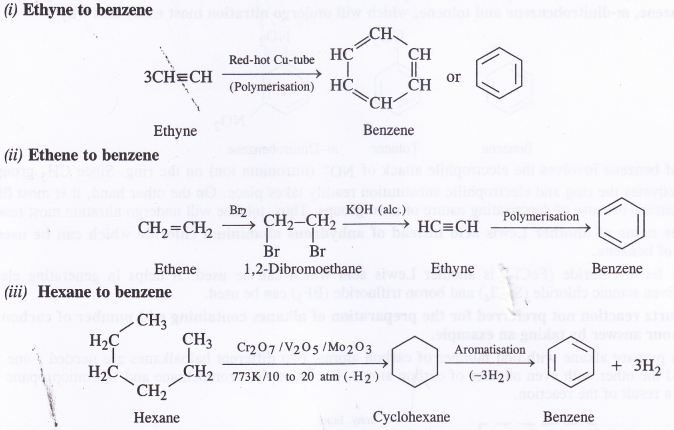
Question 21.
Write the structures of all the alkenes which upon hydrogenation give 2-methylbutane.
Answer:
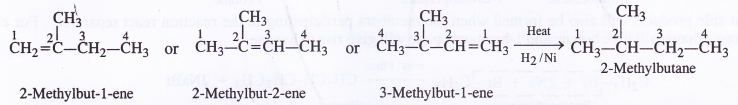
Question 22.
Arrange the following sets of compounds in order of their decreasing relative reactivity with an electrophile and assign reason.
(a) Chlorobenzene, 2, 4-dinitrochlorobenzene, p-nitrochlorobenzene.
(b) Toluene, pH2CC6H4NO2, pO2NC6H4NO2
Answer:
(a) The correct order of decreasing reactivity towards electrophilic substitution is :
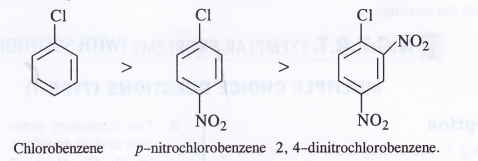
Nitro (NO2) group is a deactivating group. Its presence on the benzene ring will deactivate it towards electrophile attack since electrophile seeks a centre of high electron density. Thus, more the number of nitro groups present, lesser will be the reactivity of the compound towards electrophilic substitution.
(b) The correct order of decreasing reactivity is :
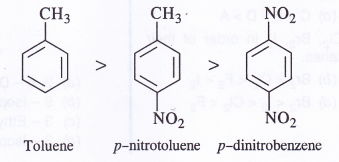
Methyl group is an activating group while the nitro group is deactivating in nature. In the light of this, the decreasing order of reactivity towards electrophilic attack is justified.
Question 23.
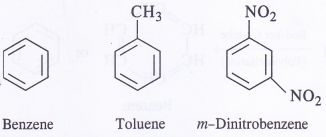
Out of benzene, /n-dinitrobenzene and toluene, which will undergo nitration most easily and why ?
Answer:
Nitration of benzene involves the electrophile attack of NO2 (nitronium ion) on the ring. Since CH3 group has +I effect, it activates the ring and electrophilic substitution readily takes place. On the other hand, it is most difficult in m-dinitrobenzene because of deactivating nature of nitro groups. Thus, toluene will undergo nitration most readily.
Question 24.
Suggest the name of another Lewis acid instead of anhydrous aluminium chloride which can be used during ethylation of benzene.
Answer:
Anhydrous ferric chloride (FeCl3) is another Lewis acid which can be used. It helps in generating electrophile (C2H5+). Even stannic chloride (SnCl4) and boron trifluoride (BF3) can be used.
Question 25.
Why is Wurtz reaction not preferred for the preparation of alkanes containing odd number of carbon atoms ? Illustrate your answer by taking an example.
Answer:
In order to prepare alkane with odd number of carbon atoms, two different haloalkanes are needed ; one with odd number land the other with even number of carbon atoms. For example, bromoethane and 1-bromopropane will give pentane as a result of the reaction.
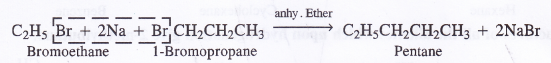
But side products will also be formed when the members participating in the reaction react separately. For example, bromoethane. will give butane and 1-bromopropane will give rise to hexane.
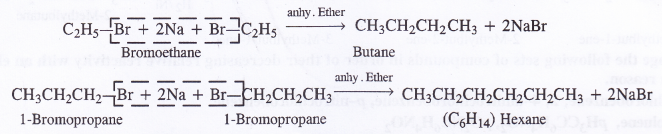
Thus, mixture of butane, pentane and hexane will be formed. It will be quite difficult to separate the individual components from the mixture.
We hope the NCERT Solutions for Class 11 Chemistry at Work Chapter 13 Hydrocarbons, help you. If you have any query regarding NCERT Solutions for Class 11 Chemistry at Work Chapter 13 Hydrocarbons, drop a comment below and we will get back to you at the earliest.