NCERT Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure
These Solutions are part of NCERT Solutions for Class 11 Chemistry. Here we have given NCERT Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure.
Question 1.
Explain the formation of chemical bond.
Answer:
Inert gases or noble gas elements are present in group 18 also called zero group. This means that their valency is zero or their atoms can exist independently of their own. Let us have a close look at their electronic configurations given in the table
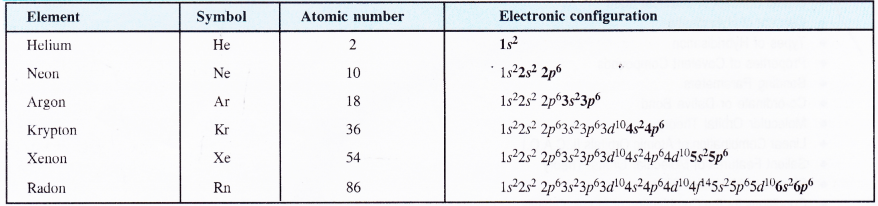
With the exception of first member helium which has only two electrons in its valence shell, the rest of the elements have eight electrons.
In 1916, G. N. Lewis and Kossel stated that the stability of noble gas elements is due to the presence of eight electrons in their valence shells (except helium) or due to the presence of complete octet. This is known as the octet rule. According to the rule, the atoms of different elements take part in chemical combination in order to complete their octet (to have eight electrons in the outermost or valence shell) or duplet (to have two valence electrons)in some cases such as H, Li, Re etc. Thus, the atoms take part in chemical combination or bond formation in order to complete their octet and to have the electronic configuration of nearest noble gas atoms.
Question 2.
Write the Lewis dot symbols of the following elements :
Be, Na, B, O, N, Br.
Answer:
Please remember that only the valence electrons are shown in the Lewis dot symbols of the atoms of the elements.
![]()
Question 3.
Write the Lewis dot symbols of the following atoms and ions :
S and S2- ; P and P3- ; Na and Na+; Al and Al3+ ; H and H–
Answer:
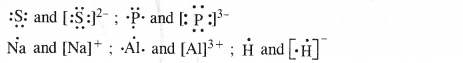
Question 4.
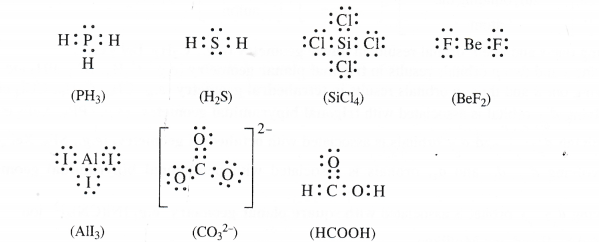
Draw the Lewis structures of the following molecules and ions :
PH3, H2S, SiCl4, BeF2, AlI3, C032-, HCOOH
Answer:
Question 5.
Define octet rule. Write its significance and limitations.
Answer:
the atoms of different elements take part in chemical combination in order to complete their octet (to have eight electrons in the outermost or valence shell) or duplet (to have two valence electrons)in some cases such as H, Li, Re etc. Thus, the atoms take part in chemical combination or bond formation in order to complete their octet and to have the electronic configuration of nearest noble gas atoms.
Significance : The octet rule i. e., the tendency of the atom of an element to acquire eight electrons in its valence shell is the basis for the formation of chemical bonds. This applies to both ionic and covalent bonds. For more details
Significance of Lewis Symbols
The Lewis symbols of the atoms have a lot of information to convey.
(a) They represent the number of valence or outermost electrons.
(b) No of valence electrons help in calculating the valency or valence of the element which is also called group valeme
i. e.„ the valence of the group to which a particular element belongs. The group valence is equal to either the number of dots in the Lewis symbol of the element or “Eight – no. of dots.” For example,
Limitations: The main limitation of the octet rule is that in certain molecules, the central atom may have either incomplete or expanded octet. But they are still quite stable. For more details,
Limitations of Octet Rule or Octet Theory
We have discussed the implication of octet rule which states that the atoms of same or different elements take part in chemica I combination to complete their octet either by transference or by sharing of electrons.
The octet rule can be applied to large number of covalent molecules, particularly those of organic nature. However, there are certain exceptions to the octet rule. These are illustrated as follows :
(a) Incomplete octet of the central atom. There are many stable covalent molecules in which the central atom has less tha t eight electrons after sharing i.e. it has an incomplete octet. For example,

(b) Old electron molecules. There are certain molecules in which even single or odd electrons may be present on th; atoms. For example,

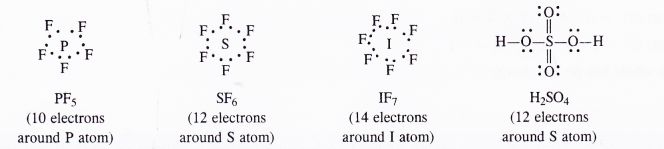
(c)Expanded octet of the central atom. There are many examples in which the central atom may have even more that eight electrons after sharing i.e. it may have expanded octet. For example,
(d) Shapes of the molecules. The octet rule or octet theory gives no idea about the, shapes of the molecules which may be tri-atomic, tetra-atomic or polyatomic in nature.
Question 6.
Write the favourable conditions for the formation of ionic bond.
Answer:
The favourable conditions for the formation of ionic bond are :
(i) Low ionization enthalpy for the element to form positive ion or cation.
(ii) High electron gain enthalpy for the element to form negative ion or anion.
(iii) High magnitude of lattice energy or lattice enthalpy.
(i). Ionization enthalpy. In the formation of positive ion or cation, one of the atoms is to lose electrons and for this ionization enthalpy is needed. As already stated, it is the amount of energy required to remove the most loosely bound electron from an isolated gaseous atom. Thus, lesser the ionization enthalpy required, easier will be the formation of cation. For example,
A(g) → A+(g) + e– ; ∆iH1 = + ve
The alkali metals and alkaline earth metals present in the s-block normally form cations since they have comparatively low ionization enthalpies.
(ii). Electron gain enthalpy. The electrons released in the formation of cation are to be accepted by the other atom . taking part in the ionic bond formation. The electron accepting tendency of an atom depends upon the electron gain
enthalpy. It may be defined as the energy released when an isolated gaseous atom takes up an electron to form anion. Greater the negative electron gain enthalpy, easier will be formation of anion. For example,
B(g) + e– → B–(g) ; ∆egH The halogens present in group 17 have the maximum tendency to form anions as they have very high negative electron gain enthalpy.
The members of the oxygen family (group 16) such as oxygen also form anions but not so easily because energy is needed t( form divalent anion (2- is comparatively high.
The details have been discussed in the unit 3 on Chemical families and periodic properties.
(iii). Lattice energy or enthalpy. The ionic compounds exist as crystalline solids and the arrangement is known as crysta lattice. Since the ions are charged species, energy known as lattice energy or enthalpy is released in the attraction o ‘ the ions. It may be defined as :
the energy released when one mole of crystalline solid is formed by the combination of oppositely charged ions.
It is denoted as U.
A+ (g) + B– (g) → A+ B– (s) ; U = – ve (Released)
Thus, greater the magnitude of lattice energy, more will be the stability of the ionic bond or ionic compound. The lattice energy depends upon the following factors :
(a) Size of the ions. The size of the ions influences the lattice energy. Smaller the size, lesser will be the internuclea distance and, thus, greater will be the lattice energy. For example, lattice energy of NaCl (769 8 kJ mol1) is mon than that of KC1 (698-7 kJ mol-1) because the radius of Na+ (95 pm) ion is smaller as compared to K+ (133 pm) ion.
(b) Charge on the ions. Greater the magnitude of charge on the ions, higher will be inter ionic attraction and thus greater will be the value of lattice energy.
Thus, we conclude that if the magnitude of lattice energy and negative electron gain enthalpy is greater than that of the ionization enthalpy required, a stable chemical bond will be formed. In case, it is less then the bond will not be formed.The Lattice Energy can be determined with the help of Born-Haber cycle. For the details, consult section 6.18 (Chapter 6 – Thermodynamics).
Question 7.
Predict the shapes of the following molecules using the V.S.E.P.R. model.
BeCl2, SiCl4, AsF5, H2S, PH3,
Answer:
The shapes of the covalent molecules can be predicted with the help of V.S.E.P.R. theory.
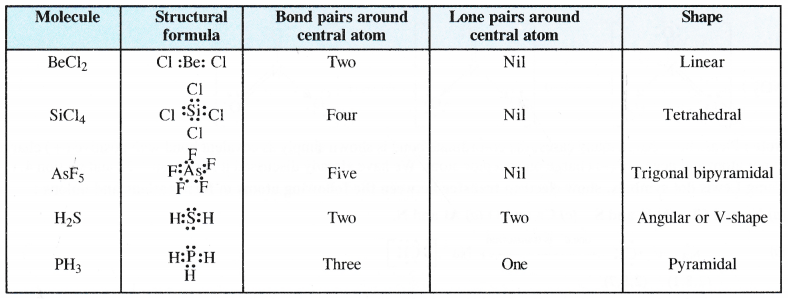
Predicting the Shapes of Covalent Molecules or Ions
The shape of a covalent molecule or ion can be predicting theoretically by calculating the total number of electron pains (shared pairs as well as lone pairs) around the central atom in the molecule or ion. This can be done by the following relation.
= 1/2[No. of valence electrons on the central atom + No. of atoms linked to the central atom by covalent bonds]
(i) For positive ion (or cation), subtract the number of electrons equal to the positive charge from the valence electrors on the central atom.
(ii) For negative ion (or anion), add the number of equal to the negative charge to the valence electrons on the central atom.
The number of bond pairs or shared pairs = No. of atoms linked to the central atom by single bonds.
The number of lone pairs = Total number of electron pairs-No. of bond pairs or shared pairs.
Question 8.
Although geometries of NH3 and H2O molecules are distorted tetrahedral, bond angle in water is less than that of ammonia. Discuss.
Answer:
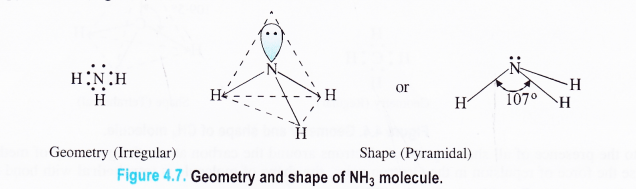
In \(\ddot { N }\)3 and in H2\(\ddddot { O } \) molecules the central atom is surrounded by four electron pairs. The geometries of these molecules are expected to be tetrahedral.
They however, get distorted on account of the presence of lone electron pairs. On comparison, the bond angle in NH3 (107°) is more than in H2O (104.5°).
This is on account of greater magnitude of force of lone pair : lone repulsion in H2O molecule as compared to lone pair : shared pair repulsion in NH3 molecule.
Ammonia molecule (NH3)
In ammonia, the atomic number (Z) of nitrogen is 7 and its electronic configuration is 2, 5. Out of the five electrons present in the valence shell of nitrogen atom, three form shared pairs (bond pairs) with the electrons of three hydrogen atoms.
The remaining two electrons constitute one lone pair.
Thus, the nitrogen atom is surrounded by three shared pairs and one lone pair of electrons. According to VSEPR theory, the geometry of the molecule is irregular.
In order to have minimum force of repulsion in all the four electron pairs around the nitrogen atom, the shape is expected to be tetrahedral but the presence of one lone electron pair distorts the shape of the NH3 molecule.
Actually, Ip – bp repulsion is greater than the bp – bp repulsion. As a result, the two N-H bonds on both sides of the lone pair are stretched a little inwards. Thus, the bond angle in the molecule is 107° and is slightly less than the bond angle of a regular tetrahedron. The actual shape of the NH3molecule is pyramidal .
Question 9.
How do we express bond strength in terms of bond order ?
Answer:
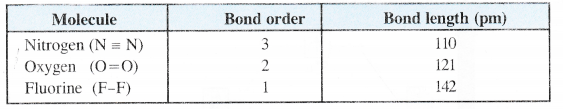
Bond strength is directly proportional to bond order, i.e., greater the bond order, more is the bond strength. For example,

Question 10.
Define bond length.
Answer:
Bond Length. Bond length may be defined as :
the average equilibrium distance between the centres of the two bonded atoms.
The bond length of different covalent bonds are determined by X-ray diffraction methods. For a covalent bond, it is the sum of covalent radii of the bonding atoms. For example, the bond length of C—Cl bond is rc + rC]. The values of the bond lengths are generally expressed in picometre (1 pm — 1(F12 m). The bond length is influenced by the following factors.
(i)Size of the atoms. As stated earlier, bond length is directly linked with the size of the bonding atoms. For example H—Br bond length (141 pm) is more than H—Cl bond (127 pm).
(ii)Multiplicity of bonds. The multiplicity of the bonds between two atoms brings them close to each other. As a result, the bond length decreases. For example, bond length for C—C bond is 154 pm and the value for C = C bond is 134 pm.
(iii)Types of hybridisation. The value of bond length is also influenced by the type of hybridisation. Since s-orbital is smaller in size as compared to the p-orbital, therefore, greater the s-character associated with a particular bond, smaller will be the bond length. For example,
sp3 C—H (111 pm) > sp2 C—H bond (110 pm) > sp C—H bond (108 pm).

Question 11.
Explain the important aspects of resonance with respect to the \({ CO }_{ 3 }^{ 2- }\) ion
Answer:
Resonance
Covalent molecules are normally expressed as Lewis structures. But in some cases, a single Lewis structure fails to explain all the characteristics of the molecule. For example, the Lewis structure of ozone is represented as follows :
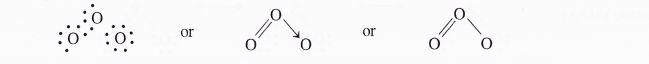
The two bonds in the molecule are expected to have different values. But the experimental studies have shown that both the bonds have the same lengths (128 pm). Moreover, the bond length is intermediate between the single and double bonds.
The problems can be solved if we consider that ozone is capable of existing in two forms in which the positions of the double bond and single bond are interchangeable.
Such structures for the molecule are known as resonating or contributing structures separated by (sign for resonance). None of these structures can independently explain all the properties of ozone.
It is believed that the actual structure is inbetween these two contributing structures and is known as resonance hybrid as
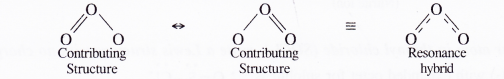
Thus, O to O bond length in the molecule is the same for both the bonds i.e. 128 pm. Resonance may be defined as :
the phenomenon as a result of which a molecule can be expressed in different forms none of which can explain all the properties of the molecule. The actual structure of the molecule is called resonance hybrid.
Question 12.
H3PO3 can be represented by the structures 1 and 2 as shown below. Can these structures be taken as the canonical forms of the resonance hybrid representing H3PO3 ? If not cite reasons for the same.
Answer:
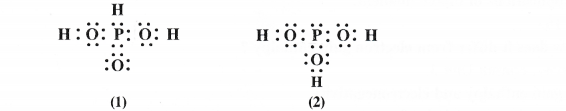
The basic requirement for the resonance is that the canonical structures must differ in the arrangement of the electron pairs and not of the atoms. The structures (1) and (2) are not the canonical structures because in structure (1) phosphorus atom is linked with H (P—H bond) while in the other (2), it is linked with OH group (P—Oil bond).
Question 13.
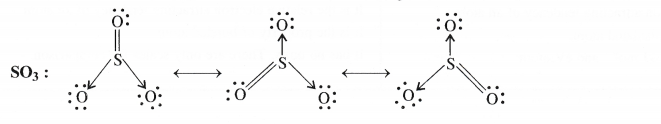
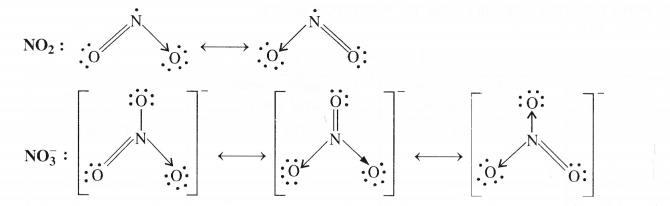
Write the resonating structures for SO3, No2 and \({ NO }_{ 3 }^{ – }\)
Answer:
Note : Please note that in many cases, the co-ordinate bond is shown simply as covalent bond with positive (+) charge on donor atom and negative (-) charge on acceptor atom.
Question 14.
Using Lewis dot symbols, show electron transfer between the following atoms to form cations and anions :
(a) Na and Cl (b) K and S (c) Ca and O (d) Al and N.
Answer:
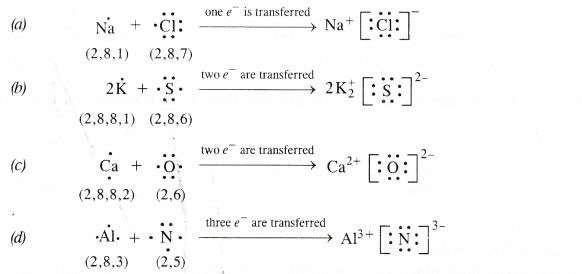
Question 15.
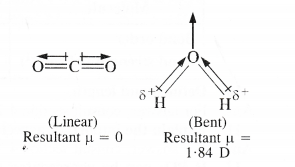
Although both CO2 and H2o are triatomic molecules, the shape of H2o molecule is bent while that of Co2 is linear. Explain this on the basis of dipole moment.
Answer:
Carbon dioxide is a linear molecule in which the two C = 0 bonds are oriented in the opposite directions at an angle of 180°. The dipole moment of C = O bond is 2.3D but due to linear structure of Co2, the bond dipoles of two C = O bonds cancel with each other.
Therefore, the resultant dipole moment of the molecule is zero. On the other hand, H20 is a polar molecule having dipole moment 1.84 D. Actually H2o molecule has a bent structure in which the O—H bonds are oriented at an angle of 104.5° and do not cancel the bond dipole moments of each other.
The molecular dipole moment of H2o (p = 1.84 D) is the resultant of the individual values of the bond dipole moment of two O—H bonds.
Question 16.
Write the significance and applications of dipole moment.
Answer:
Applications of Dipole Moment
Some useful applications of dipole moment are given :
(i) In predicting the nature of the molecules. Molecules with specific dipole moments are polar in nature while those with zero value are non-polar. Thus, BeF2 (μ= 0D) is non-polar while H20 (μ = 1.84 D) is polar.
(ii) In comparing the relative polarities of the molecules. The relative polarities of the molecules can be compared by their dipole moment values. Greater the value, more is polarity.
(iii) In determining the shapes of molecules. The dipole moment values are quite helpful in determining the general shapes of the molecules having three or more atoms. In case such molecules have dipole moments, then their shapes will not be symmetrical.
They will be either bent or angular, (e.g. H2S with// = 0.90 D) or unsymmetrical (e.g. NH3 with// = 1.47 D). However, for molecules with zero dipole moment values, the shapes will be either linear (BeF2, Co2 etc.) or symmetrical (CH4, CCl4 etc.)
(iv) In calculating the percentage ionic character. The dipole moment values help in calculating the percentage ionic characters of polar bonds. It is the ratio of the observed dipole moment or experimentally determined dipole moment to the dipole moment for the complete transfer of electrons.
For example, the observed dipole moment of HCl molecule is 1.03 D. For complete transfer of electrons (100% ionic character) the charge on H+ and Cl– ions would be equal to one unit (4.8 × 10-10 esu) each. The bond length of H—Cl bond is 1.275 × 10-8 cm. Therefore,
Dipole moment for complete electron transfer (μionic) = q × d
= (4.8 × 10-10esu) × (1.275 × 10-8 cm)
= 6.12 × 10-18 esu-cm = 6.12D
Observed dipole moment (μobserved) = 1.03 D
![]()
In a similar manner, the percentage ionic character for other polar bonds can also be calculated.
(v) In distinguishing between cis and trans geometrical isomers. In organic compounds, dipole moment can halp in making a distinction between the cis and trans geometrical isomers of a substance. In general, cis isomer (less symmetrical) has more dipole moment value as compared to the trans isomer. For example, the dipole moment cis-but-2-ene is higher than that of trans-but-2-ene.
(vi) In identifying the position of the substituents in the disubstituted aromatic rings. The relative positions of the substituents such as methyl (- CH3) groups present at ortho, meta and para positions can be identified from their dipole moments. The dipole moment of para-isomer is zero. Out of the ortho and meta isomers, the dipole moment of the ortho iosmer is higher.
Question 17.
Define electronegativity. How does it differ from electron gain enthalpy ?
Answer:
Difference between electron gain enthalpy and electronegativity.
The main points of distinction are given in a tabular form.

Question 18.
Explain with the help of suitable example polar covalent bond.
Answer:
We have studied that according to Lewis concept, a covalent bond represents a shared pair of electrons. If the atoms joined by covalent bond are the same then the shared electron pair is equidistant to both of them. In other words, it lies exactly in th: centre of the bonding atoms. As a result, no poles are developed and the bond is called non-polar covalent bond. Th: corresponding molecules are known as non-polar molecules. For example,
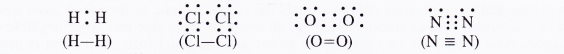
On the other hand, in case different atoms are linked to each other by covalent bond, then the shared electron pair will not lie in the centre because the bonding atoms differ in electronegativities.
For example, in hydrogen chloride molecule, chlorine with greater electronegativity (3.0) will have greater control over the electron pair as compared to hydrogen which is less electronegativ : (2.1). Since the electron pair is displaced more towards chlorine atom, the latter will acquire a partial negative charge (δ-).
At the same time, the hydrogen atom will have a partial positive charge (δ + ) with the magnitude of charge same as on the chlorine atom. Such a covalent bond is known as polar covalent bond or simply polar bond and is represented as follows :

The molecule with such a bond is called polar covalent molecule. It may be noted that greater the difference in the electronegativity of the bonding atoms, more will be the polarity of the bond. For example,
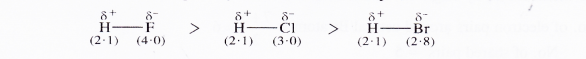
Question 19.
Arrange the bonds in order of increasing ionic character in the molecules :
LiF, K2o, N2, SO2 and ClF3
Answer:
N2 < SO2 < ClF3v < K2o < LiF.
Question 20.
The skeletal structure of CH3COOH as shown below is correct but some of the bonds are wrongly shown. Write the correct Lewis structure of acetic acid.
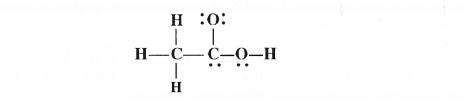
Answer:
Only the skeletal arrangement of the atoms in the above structure is correct. But it is not according to the Lewis concept as well as tetravalent nature of carbon. The correct structure of acetic acid is
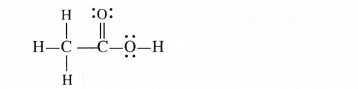
Question 21.
Apart from tetrahedral geometry for methane (CH4), another possible geometry is square planar with four ‘FT atoms at the comers of the square with the ‘C’ atom at its centre. Explain why CH4 is not square planar.
Answer:
The tetrahedral and square planar structures of CH4 are shown as follows :
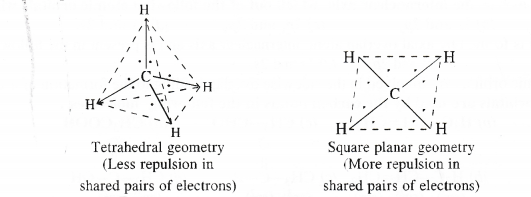
According to VSEPR theory, the shared electron pairs around the central atom in a covalent molecule are so arranged in space that the force of repulsion in them is the minimum. Now, in a square planar geometry, the bond angle is 90° while it is 109°-28′ in tetrahedral geometry. This clearly shows that the electron repulsions are less in the tetrahedral geometry as compared to the square planar geometry. Thus, methane cannot be represented by square planar structure.
Question 22.
Explain why BeH2 molecule has zero dipole moment although the Be—H bonds are polar.
Answer:
BeH2 is a linear molecule (H—Be—H) with bond angle equal to 180°. Although the Be—H bonds are polar on account of electronegativity difference between Be (1.5) and FI (2.1) atoms, the bond polarities cancel with each other. The molecule has resultant dipole moment of zero.
Question 23.
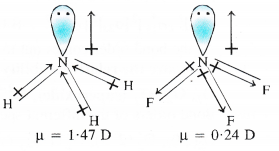
Both NH3 and NF3 have identical shapes and same state of hybridisation. Both N—H and N—F bonds have almost the same electronegativity difference. But still, the two molecules have different dipole moment values. How will you account for it ?
Answer:
Both NH3 and NF3 have pyramidal geometries and the electronegativity difference of bonding atoms in both NH3 (3.0 -2.1= 0.9) and NF3 (4.0 – 3 0 = 1.0) molecules are also nearly the same. But the dipole moment of NH3 (1.46D) is more than that of NF3 (0.24D).
This is explained on the basis of the difference in the directions of the dipole moments. In NH3, the dipole moments of the three N—H bonds are in the same direction as that of the lone electron pair.
But in NF3, the dipole moments of the three N—F bonds are in the direction opposite to that of the lone pair. Therefore, the resultant dipole moment in NH3 is more than in NF3.
Question 24.
What is meant by hybridisation of atomic orbitals ? Describe the shapes of sp, sp2 and sp3 hybrid orbits.
Answer:
Hybridisation is of different types depending upon the nature and number of orbitals taking part in hybridisation. Let us discuss the hybridisation involving 5, p and d orbitals.
Hybridisation involving s and p-orbitals
The hybridisation involving 5 and p orbitals is of three types depending upon the orbitals that are involved. These are sp3, sp2 and sp types :

Question 25.
What is meant by the hybridisation of atomic orbitals ? What is the change in hybridisation (if any) of A1 atom in the reaction ?
AlCl3 + Cl– → AlC\({ l }_{ 4 }^{ – }\).
Answer:
Hybridisation is of different types depending upon the nature and number of orbitals taking part in hybridisation. Let us discuss the hybridisation involving 5, p and d orbitals.
In AlCl3, the central Al atom is sp2 hybridised while in the ion [AlCl4]-, it has sp3 hybridisation.
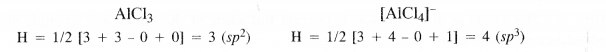
Question 26.
Is there any change in the hybridisation of B and N atoms as a result of the following reaction ?
BF3 + :NH3 → F3B.NH3
Answer:
In BF3, B atom is sp2 hybridised. It forms an addition compound with \(\ddot { N }\)3 (Where N atom is sp3 hybridised). In doing so, it takes up an electron pair from NH3. This indicates that B atom undergoes a change in hybridisation from sp2 to sp3. At the same time there is no change in hybridisation of N atom.
Question 27.
Draw diagrams showing the formation of a double and a triple bond between the carbon atoms in the (C2H4 and C2H4molecules.
Answer:
Formation of ethene (CH2 = CH2). In order to illustrate sp2 hybridisation, let us discuss the orbital structure of ethene (H2C = CH2) molecule. Out of three hybridised orbitals belonging to each carbon atom, one is involved in the axial overlap with the similar orbital of the other carbon resulting in a sigma bond.
The remaining two hybridised orbitals of the carbon atoms form sigma bonds with the lx orbital of the hydrogen atoms. The unhybridised orbitals (2pz) of both the carbon atoms participate in the sidewise overlap leading to a pi (π) bond as shown in the Fig. 4.27.
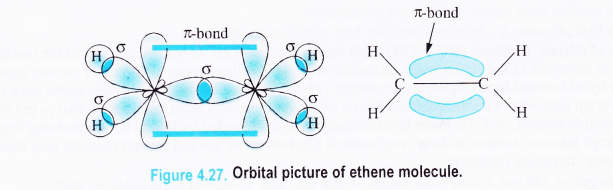
Question 28.
What is the total number of sigma and pi bonds in the following molecules ?
(a) C2H2 (b) C2H4
Answer:
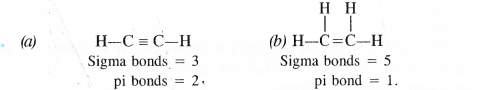
Question 29.
Considering X-axis as the internuclear axis, which out of the following atomic orbitals will form a sigma bond ?
(a) 1s and 1s
(b) 1s and 2px
(c) 2py and 2py
(d) 1s and 2s.
Answer:
The sigma bond is formed by axial overlap along internuclear axis and is present in the following cases.
(a)1s and 1s
(b) 1s and 2px
(d) 1s and 2s.
2py and 2py atomic orbitals are involved in the sidewise overlap leading to the formation of π-bond.
Question 30.
Which hybrid orbitals are used by the carbon atoms in the following molecules ?
(a) H3C—CH3
(b) H3C—CH=CH2
(C) CH3—CHO
(d) CH3COOH
Answer:
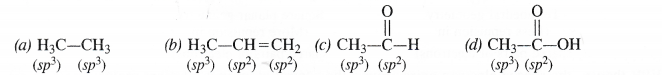
Question 31.
What do you understand by bond pairs and lone pairs of electrons ? Illustrate by giving one example of each type.
Answer:
The electron pair involved in sharing between two bonding atoms is called shared pair or bond pair. At the same time, the electron pair which is not involved in sharing is called lone pair of electrons. For example, in BeH2 molecule, the central Be atom is surrounded by two bond pairs. In the molecule of NH3, the central B atom has three shared pairs and one lone pair around it.
Question 32.
Distinguish between a sigma and a pi bond.
Answer:
Types of Orbital Overlap (Sigma and Pi Bonds)
We have studied that the covalent bonds are formed by the overlap of the atomic orbitals. Depending upon the type of the overlapping, the covalent bonds are of two types known as sigma (\(\sigma \) ) and pi (π) bonds. These are described as below :
Sigma (\(\sigma \) ) bond. A sigma bond is formed when the two half filled atomic orbitals belonging to the bonding atoms overlap along their internuclear axes i.e. the line joining the centres of the nuclei of the two atoms.
Thus, the bond formed as a result of the axial overlap is known as \(\sigma \) bond. Since the axial overlap is quite large, the energy released during the overlap is also large. As a result, the sigma bond is a stable bond.
In most of the covalent molecules, s and p orbitals are involved in the bond formation. The axial overlap involving these orbitals is of three types :
(a) s-s overlap. This involves the axial overlap of the half filled v-atomic orbitals of the combining atoms. The cr-bond is
called s-s bond.
(b) s-p overlap. This involves the overlap of the s-atomic orbital belonging to one atom and p-atomic orbital to the other atom along their axes. The bond is known as s-p bond.
(c) p-p overlap. In this case, the atomic orbitals taking part in the axial overlap are both half filled p-orbitals. The \(\sigma \) -bond formed is called p-p bond.
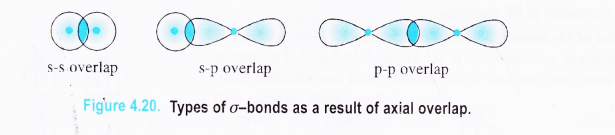
Question 33.
Explain the formation of H? molecule on the basis of valence bond theory.
Answer:
Formation of hydrogen (H2) molecule
In the light of the above discussion, let us consider the combination between atoms of hydrogen HA and HB. If eA and eB be their respective electrons, then attractive and repulsive forces may be shown in the Fig. 4.9.
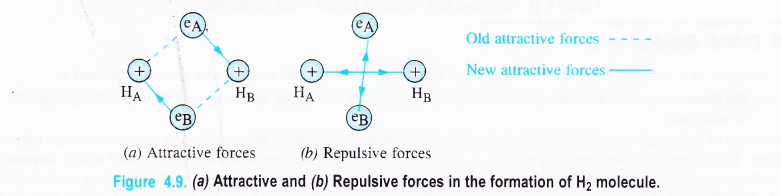
Although the number of new attractive and repulsive forces is the same, but the magnitude of the attractive forces is more. Thus, when two hydrogen atoms approach each other the overall potential energy of the system decreases. Therefore, a stable molecule of hydrogen gets formed.
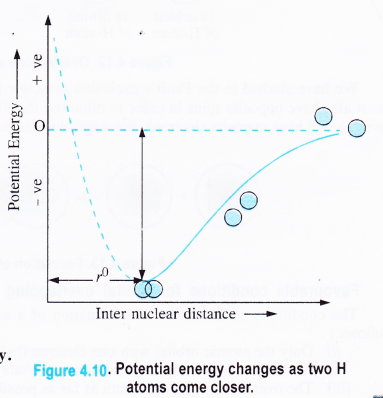
The potential energy changes which take place in the formation of a hydrogen molecule may also be shown graphically as follows: When the two hydrogen atoms are far separated (at infinite distance) the potential energy is zero. As they start coming closer to each other from infinite distance, energy is lost correspondingly.
Ultimately, a point of minimum energy is attained when the attractive and repulsive (or maximum stability) forces balance each other. At this stage, the two hydrogen atoms are bonded together to form a molecule of hydrogen.
M The distance between the centres of their nuclei is called bond length. In c case of hydrogen molecule, H—H bond length is 74 pm. It may be kept — in mind that the two hydrogen atoms can not be brought closer than 74 pm.
If it happens, then the repulsive forces will become more than the 3 attractive forces. As a result, the potential energy of the system will increase as shown by the dotted line.
The total decrease in the potential energy when one mole bonds of a particular type are formed between the atoms in the gaseous state is called bond energy. For example, the bond energy of H—H(g) is 433 kJ mol-1.
It may be noted that the same energy is needed to break the molecules into the atoms and is known as bond dissociation energy. Thus, the bond dissociation energy of hydrogen is also 433 kJ mol-1.
Question 34.
Write the important conditions required for the linear combination of atomic orbitals to form molecular orbitals.
Answer:
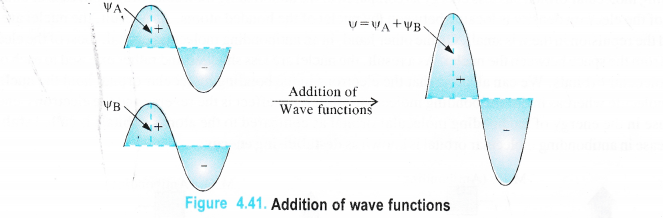
The molecular orbitals are formed by the linear combination of the wave functions of the participating atomic orbitals. They may combine either by addition or by subtraction. Let yA and yB represent the wave functions (or amplitude) of the two combining atomic orbitals A and B taking part in chemical combination.
(a) Combination by addition. When the two electron waves are in phase i.e. they have the same sign*, they will add up to give a new wave function expressed as φ (or \(\phi \)) = φA +φB.
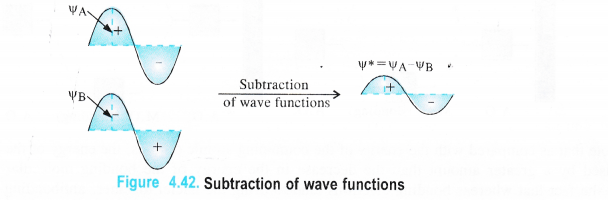
(b) Combination by subtraction. When the two electron waves are out of phase i.e. they have opposite sign of the wave functions, then they will combine by subtraction and the resulting wave function φ* (or <()*) = φA – φB as shown below :
We know that the probable electron density is given by φ2 and not by φ. On squaring we get :
φ2 = φA2 + φB2 + 2φAφB
φ*2 = φA2 + φB2 – 2φAφA
Here yA2 and yB2 represent the probable electron density in the two atomic orbitals whereas φ2 and φ*2 indicate the electron density in the two molecular orbitals. These are called Bonding Molecular orbital (φ2) and Antibond ing Molecular orbital (φ*sup>2).
• Bonding Molecular Orbital (φ2) is formed by the linear combination of wave functions of the combining atomic orbitals [L.C.A.O) by addition.
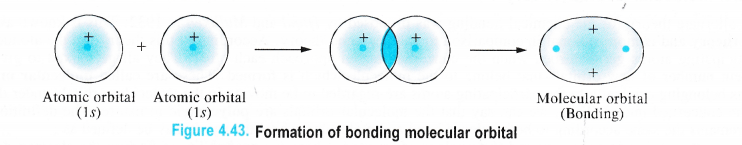
• Antibonding Molecular Orbital (φ*sup>2) is formed by the linear combination of wave functions of the combining atomic orbitals (L.C. A. O) by subtraction. Let us illustrate both these orbitals by the combination of the atomic orbitals of hydrogen atoms
The formation of bonding molecular orbital when two atomic orbitals of hydrogen atoms (Is orbital) combine by the addition of their wave functions is shown below :
Similarly, the antibonding molecular orbital arising from the subtraction of the wave functions of the two participating atomic orbitals is shown as follows :
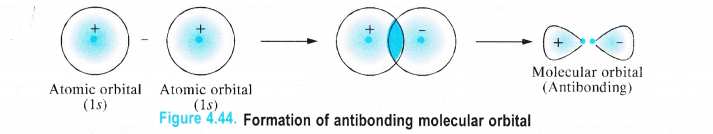
Thus we conclude that whenever two atomic orbitals combine, two molecular orbitals are formed. One of them is bonding molecular orbital (φ2M 0) while the other is antibonding molecular orbital (φ*2M.O)-
Question .35.
Use molecular orbital theory to explain why Be2 molecule does not exist.
Answer:
The atomic no. (Z) of Be is 4. This means that 8 electrons are to be filled in the M.O of Be2. The configuration is :
![]()
As the bond order comes out to be zero, the molecule of Be2 does not exist.
Question 36.
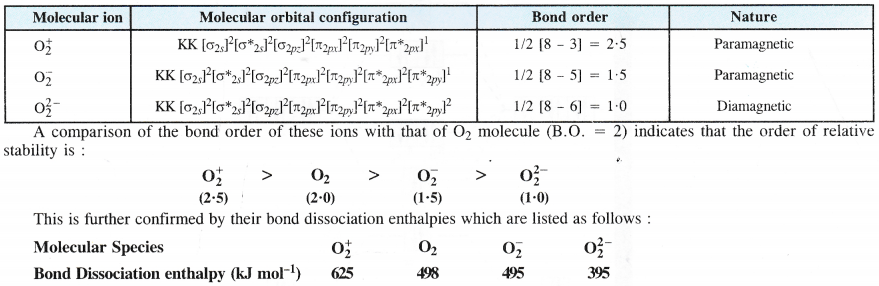
Compare the relative stability of the following species and indicate their magnetic properties :
O2, \({ O }_{ 2 }^{ + }\),\({ O }_{ 2 }^{ – }\) (superoxide), \({ O }_{ 2 }^{ 2- }\) (peroxide)
Answer:
Bond orders of the different species are ;
O2(2.0), \({ O }_{ 2 }^{ + }\)(2.5), \({ O }_{ 2 }^{ – }\)(l.5), \({ O }_{ 2 }^{ 2- }\)(1.0)
Relative stability : \({ O }_{ 2 }^{ + }\) > O2 > \({ O }_{ 2 }^{ – }\) > \({ O }_{ 2 }^{ 2- }\)
For magnetic properties; consult section 4.32.
Question 37.
Write the significance of plus and minus signs in representing the orbitals.
Answer:
Plus and minus signs have been given to identify the nature of the electron waves. Plus (+ve) sign denotes crest while negative (-ve) sign denotes trough.
Question 38.
Describe the hybridisation in case of PCl5. Why are the axial bonds longer as compared to equatorial bonds ?
Answer:
The hybridisation in the molecule of PCl5 is same as in PF5 because the central atom is the same in both the molecules and the two halogens chlorine (Cl) and fluorine (F) also belong to the same group (group 17). They are expected to have same hybridisation as well as same geometry. The P atom is sp2d hybridised and the molecule has trigonal bipyramidal geometry. The details of hybridisation of PF5
Question 39.
Define hydrogen bond. Is it weaker or stronger than the van der Waals forces ?
Answer:
Hydrogen bond represents strong dipole dipole interactions between the H and the highly electronegative atoms (F, O and N) present in different bonds belonging to either different molecules or same molecule van der Waals forces are also dipole forces but are comparatively weak. For the details of hydrogen bond, consult section 4.34. For the details of van der Waals, forces, consult unit-5 on States of Matter.
Question 40.
What is meant by the term bond order ? Calculate the bond order of: N2, O2, \({ O }_{ 2 }^{ – }\) and \({ O }_{ 2 }^{ 2- }\); .
Answer:
Significance of Bond Order. The bond order conveys very important information about the molecules and ions. These are briefly discussed.
(a) Stability of molecules or ions. The stability of the molecules and their ions can be predicted in terms of bond order. If bond ‘ order is positive, (Nb > Na), the molecule or ion will be stable. Incase, it is zero (Nb = Na) or negative (Nb< Na), the molecule or ion will be unstable. A fractional value of the bond order suggests that the molecule is unstable. However, it still exists.
(b) Bond dissociation enthalpy. Bond dissociation enthalpy is directly proportional to the bond order. Thus, more the value of bond order, greater will be the bond dissociation enthalpy and vice versa. This is further supported by the available data.
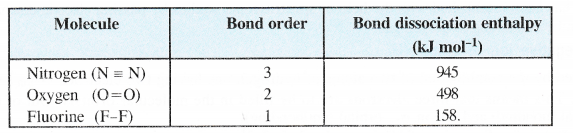
(c) Bond Length. Bond order is inversely proportional to bond length. This means that greater the bond order, smaller will be the bond length.
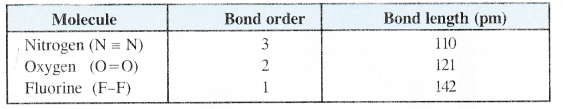
(d) Number of bonds. The value of bond order also predicts the number of covalent bonds in the molecule. Bond order 1, 2, 3 signifies the presence of single, double and triple bond respectively in a particular molecule, However, in some cases, the bond order is fractional (e.g. 0-5) and it is quite difficult to explain its existence.
We hope the NCERT Solutions for Class 11 Chemistry at Work Chapter 4 Chemical Bonding and Molecular Structure, help you. If you have any query regarding NCERT Solutions for Class 11 Chemistry at Work Chapter 4 Chemical Bonding and Molecular Structure, drop a comment below and we will get back to you at the earliest.