NCERT Solutions for Class 11 Chemistry Chapter 9 Hydrogen
These Solutions are part of NCERT Solutions for Class 11 Chemistry. Here we have given NCERT Solutions for Class 11 Chemistry Chapter 9 Hydrogen
Question 1.
Justify the position of hydrogen in the periodic table on the basis of its electronic configuration.
Answer:
Position of Hydrogen in the Periodic Table
In the periodic table, hydrogen has been placed at the top of the alkali metals in group 1 although it is not a member of the group. However, its position is not properly justified simply because of its electronic configuration (Is1). It can be placed along with the alkali metals as they too have ns1 configuration. However, it can also be placed along with halogens in group 17 since just like halogens, it also requires any one electron to have the configuration of the nearest noble gas element helium. Let us compare its characteristics with the members of both the families
Resemblance with Alkali metals
Hydrogen and alkali metals resemble in the following respects.
Electronic configuration. Hydrogen has one electron in its valence shell like the alkali metals. For example,
Element H Li Na
Atomic No. 1 3 11
Electronic configuration Is1 1s22s’ ls22s22p63s1
Question 2.
Write the names of the three isotopes of hydrogen. What is the mass ratio of these isotopes ?
Answer:
The three isotopes of hydrogen are : protium (\( _{ 1 }^{ 1 }{ H }\)) deuterium( \( _{ 1 }^{ 2 }{ H }\)or D) and tritium ( \( _{ 1 }^{ 3 }{ H }\) or T). Mass ratio of the three isotopes is :
![]()
Question 3.
Why does hydrogen occur in diatomic form rather than in monoatomic form under normal conditions ?
Answer:
In monoatomic form, hydrogen atom has only one electron in K-shell (Is1) while in diatomic form, the K-shell is complete (Is2). This means that in diatomic form, hydrogen (H2) has acquired the configuration of noble gas helium. It is therefore, quite stable.
Question 4.
How can the production of dihydrogen from ‘coal gasification’ be increased ?
Answer:
In coal gasification reaction, steam vapours are passed through coke to form mixture of CO and H2 also called syn gas.
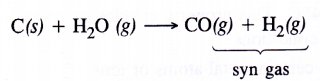
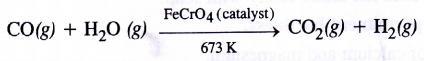
The production of dihydrogen can be increased by reacting CO(g) present in syn gas with steam in the presence of iron chromate catalyst With the removal of CO(g) or CO2(g), the reaction shifts in the forward direction. This is known as water gas shift reaction. As a result, the production of dihydrogen will increase
Question 5.
Describe the bulk preparation of dihydrogen by electrolytic method. What is the role of an electrolyte in the process ?
Answer:
Dihydrogen is formed mainly from water by carrying its electrolysis in the presence of suitable electrolytes i.e., small amount of an acid or alkali. Actually, water as such is a poor conductor of electricity. Addition of small amount of electrolyte increases its conductivity.
Question 6.
Complete the following
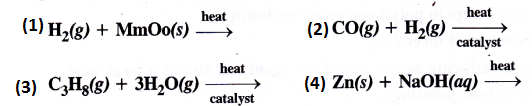
Answer:
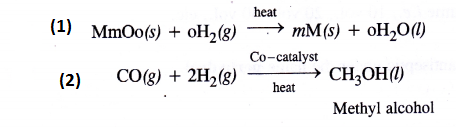
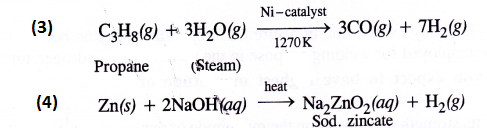
Question 7.
Describe the consequences of high bond enthalpy of H-H bond in terms of chemical reactivity of dihydrogen.
Answer:
The bond enthalpy or bond dissociation enthalpy of dihydrogen (H2) is very high (AH = 435 -9 kJ mol-1). This is on account of its small atomic size and also small bond length (74 pm) of H—H bond. Consequently, the bond cleavage is extremely difficult and molecular hydrogen or dihydrogen takes part in chemical reactions only under specific conditions.
Question 8.
What do you understand by
(1) electron deficient
(2) electron precise and
(3) electron rich compounds of hydrogen ? Provide justification with suitable examples.
Answer:
(1) Electron deficient hydrides : These hydrides have lesser number of electrons available for writing their conventional structures according to Lewis concept. One popular example of the hydrides belonging to this class is of diborane (B2Hg). No electrons are available to account for the bonding between the two atoms of boron. We shall discuss the details about the structure of diborane in unit-11 onp-block elements. Most of the hydrides of elements of group 13 fall under this category. This electron deficient hydrides may be regarded as Lewis acids and are electron acceptors.
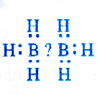
(2) Electron precise hydrides : These are the covalent hydrides in which the atoms have the required number of electrons which are permissible according to Lewis concept of bond formation. Hydrocarbons like methane (CH4), ethane (C2Hg), ethene (C2H4) and ethyne (C2H2) etc. are electron precise hydrides. We shall discuss them in unit-13.
(3) Electron rich hydrides : In these hydrides, the participating elements donot have the required number of hydrogen atoms. As a result, they have one or more lone pairs of electrons present. The elements generally belong to groups 15, 16 and 17 A few important of out of these are
![]()
They have one, two and three lone pairs of electrons present one N, O and F atoms respectively. Due to the presence of lone electron pairs, these are expected to behave as Lewis bases. However, except for NH3, both H2O and HF hardly exhibit basic nature of oxygen and fluorine. However, all the three hydrides which are listed, participate in intermolecular hydrogen bonding and get associated due to strong polar character.
Question 9.
What characteristics do you expect from an electron deficient hydride with respect to its structure and chemical reaction ?
Answer:
It is expected to be a Lewis acid. For example, diborane B2H6 (dimer of BH3) forms a complex with LiH which gives H– ion (Lewis base)
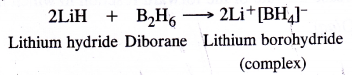
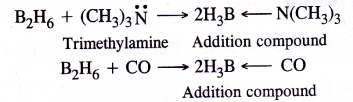
Similarly it also combines with trimethylamine (organic compound) and carbon monoxide which act as Lewis base to form addition compounds.
Question 10.
Do you expect the carbon hydride of the type (CnH2n+2) to act as Lewis acid or base ? Justify you answer.
Answer:
It is neither a Lewis acid nor a Lewis base. In all the hydrides belonging to this type, the carbon atoms have complete octet. Therefore, these hydrides behave as normal covalent hydrides also called saturated hydrocarbons or alkanes, (e.g., CH4, C2H6, C3H8 etc.). These are also called electron precise hydrides. We shall study in details about these hydrides at a later stage in unit 13.
Question 11.
What do you understand by the term non-stoichiometric hydrides ? Do you expect these types of hydrides to be formed from alkali metals ? Justify your answer.
Answer:
Metallic or Non-stiochjometric (or Interstitial) Hydrides
Many transition and inner transition metals absorb hydrogen into the interstices of their lattices to yield metal like hydrides also called interstitial hydrides. The hydrogen is present in the atomic form in these hydrides. It is interesting to note that the transition metals of groups 3, 4 arid 5 form metallic hydrides. In group 6, chromium alone has a tendency to form hydride CrH. Then there is a gap and the metals of groups 7, 8 and 9 donot form hydrides. This is known as hydride gap. Latest studies have shown that only the hydrides of Ni, Pd, Ce and Ac are interstitial in nature which means that hydrogen atoms occupy the interstitial sites.
The rest of the hydrides have different lattices and it is not proper to call them interstitial hydrides. The elements of/-block form limited number of hydrides. The hydrides are generally non-stoichiometric and their composition vary with temperature and pressure. For example, TiH1.73, CeH2.7, LaH2.8 etc. These hydrides have metallic appearance and their properties are closely related to those of the parent metal. Most of them are strong reducing agents probably due to the presence of free hydrogen atoms in metal lattice. Metallic hydrides can be used for storing hydrogen Alkali metals do not form these type of hydrides because in their crystal lattices, atoms of hydrogen, which are very small in size do not fit in.
Question 12.
How would you expect the metallic hydrides to be useful for hydrogen storage ? Explain.
Answer:
Some metals like palladium (Pd), platinum (Pt) etc. have a capacity to adsorb large volume of hydrogen on their surface forming hydrides. In fact, hydrogen dissociates on the surface of metal as H atoms which are adsorbed. In order to accommodate these atoms, the metal lattice expands and becomes rather unstable upon heating. The hydride releases hydrogen and changes back to the metallic state. The hydrogen evolved in this manner can be used as a fuel. The metals listed above (belonging to transition metals) can be used to store as well as transport hydrogen which is to be used as a fuel. Thus, metal hydrides have a very useful role in hydrogen economy.
Question 13.
How does atomic hydrogen or oxy-hydrogen torch function for cutting and welding purposes ? Explain.
Answer:
Atomic hydrogen torch.When molecular hydrogen is passed through tungsten electric arc (2000 – 3000°C), at low pressure, it dissociates to form atoms of hydrogen, known as atomic hydrogen.
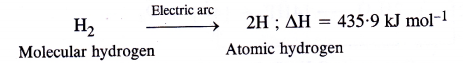
Atomic hydrogen is extremely unstable (life period is about 0-3 sec). Therefore, hydrogen atoms immediately unite to form molecular hydrogen again accompanied by the liberation of a large amount of heat energy. The temperature rises to 4000 – 5000°C. This is the principle of atomic hydrogen torch which is used for welding purposes. oxy-hydrogen torch.
When hydrogen is burnt in oxygen, the reaction is highly exothermic in nature. A temperature ranging between 2800c to 4000°C is generated. This temperature can be employed for welding purpose in the form of oxy-hydrogen torch.
Question 14.
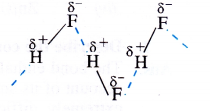
Among NH3, H2O and HF, which would you expect to have highest magnitude of hydrogen bonding and Why ?
Answer:
Hydrogen bonding represents dipolar attraction. Its strength depends upon the magnitude of the polarity of the bond. The electro negativities of three non-metals F, O and N involved in these compounds are 4, 3-5 and 3 respectively. This means that H—F bond is maximum polar and as a result, highest magnitude of hydrogen bonding is HF molecules.
Question 15.
Saline hydrides are known to react violently with water producing fire. Can CO2, a well known extinguisher, be used in this case ? Explain.
Answer:
Whenever a saline hydride (NaH or CaH2) reacts with water, the reaction is so highly exothermic that the hydrogen evolved catches fire. For example,
NaH(s) + H2O(aq) → NaOH(aq) + H2(g) + heat
CaH2(s) + 2H2O(aq) → Ca(OH)2(aq) + 2H2(g) + heat
CO2 which is normally used as fire extinguisher cannot be used in this case because it will react with the hydroxide formed in the reaction to form a carbonate. This will increase the rate of the forward reaction in which heat is evolved.
2 NaOH(aq) + CO2(g) →Na2CO3(aq) + H2O(aq).
Question 16.
Arrade the following:
(1) IH, NaH and CsH in order of increasing ionic character.
(2) Hlr-H, D—D, and F—F in order of increasing bond dissociation enthalpy.
(3) N41, MgH2 and H2O in order of increasing reducing power.
Answer:
(1) Increasing ionic character: LiH < Nail < CsH.
Reason : The ionisation enthaiphy decreases in the order Li > Na> Cs. This influences the ionic character adversely which increases as shown
(2) Increasing bond dissociation enthalpy : F—F < H—H < D—D.
Reason : The bond dissociation enthalphy of :F—F: fluorine is very small (242-6 kJ mol-1) due to the repulsion in the lone pairs of electrons present on the two F atoms. Out of H2 and D2, the bond dissociation enthalphy of H—H (435-88 kJ mol-1) is less than that of D—D (443-35 kJ mol-1).
(3) Increasing reducing power : H2O < MgH2 < NaH.
Reason : NaH being ionic in nature is the strongest reducing agent. Both H2O and MgH2 are covalent in nature but bond dissociation enthalpy of H2O is higher. Therefore, it is a weaker reducing agent than MgH2.
Question 17.
Compare the structures of H2O and H2O2
Answer:
The structure of H2O is angular while that of H2O2 is non-linear just like two opposite open pages of a book. For actual structure of H2O,
Structure of water (H2O).
H2O is a covalent molecule in which the two hydrogen atoms are linked to the oxygen atoms by single covalent bonds. The oxygen atom is surrounded by two shared pairs with hydrogen atoms and it has also two lone pairs of electrons. To have a minimum force of repulsion in the four electron pairs around the oxygen atom, the structure of H2O molecule is expected to be tetrahedral.
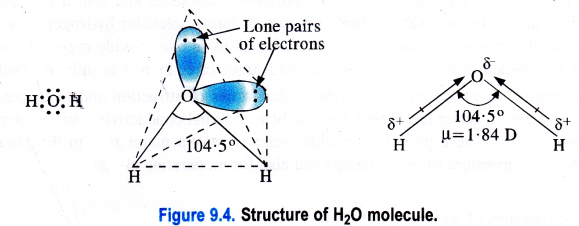
However, the two lone pairs distort its geometry and its bond angle (104-5°) is less than the bond angle of regular tetrahedron (109-28°). As the oxygen atom is more electronegative (3-5) than the hydrogen atom (2-1), therefore, both the O—H bonds are polar in nature. Because of the unsymmetrical nature of H2O molecule, the bond polarities do not cancel. Therefore, it is polar with dipole moment (ju) equal to 1 -84 D.
Structure of Hydrogen Peroxide
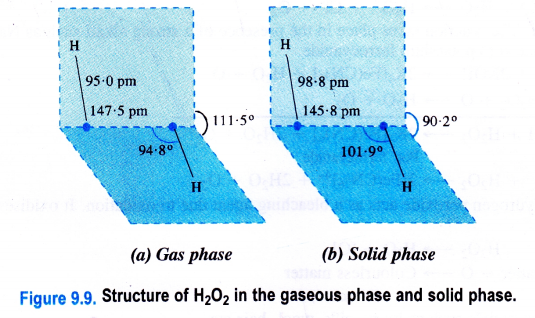
Hydrogen peroxide is a dihydroxy compound (H-O-O-H) and the 0-0 linkage is known as a peroxide linkage. It is a nonlinear molecule as the two 0-H bonds are in different planes. The interplanar (dihedral) angle is 111-5° in the gaseous phase but is reduced to 9p-2° in the crystalline state because of hydrogen bonding. The molecular dimensions of hydrogen peroxide in the gas and solid phases are shown.
Question 18.
What do you understand by ‘auto-protolysis’ of water ? What is its significance ?
Answer:
Auto-protolysis of water means ‘self-ionisation’ which proceeds as follows :
![]()
Because of auto-protolysis, water behaves both as a Bronsted acid and Bronsted base i.e. it is amphoteric in nature
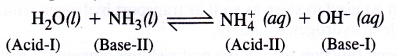
Question 19.
Consider the reaction of water with F2 and suggest in terms of oxidation and reduction which species are oxidised/reduced.
Answer:
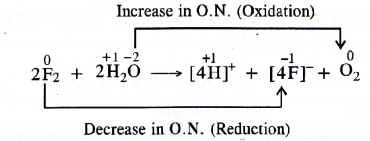
- Water (H2O) is oxidised to O2
- Fluorine (F2) is reduced to F– ions or HF
Question 20.
Complete the following :
(1) PbSfs) + H2O2(aq)→
(2) MnO4(dq) + H2O2(aq) + H+(aq) →
(3) CaO(s) + H2O(g)→
(4) AlCl3(s) + H2O(l) →
(5) Ca3N2(s) + H2O(l) →
Answer:
- PbS(s)+ 4H2O2(aq) →PbSO4(.s) + 4U2Oaq)
- 2MnO–4 (aq) + 6iA+(aq) + 5R2O2(aq) → 2Mn2+(aq) + 8H2O(l) + 5O2(g)
- CaO(s) + H2O(g) → Ca(OH)2(s)
- AlCl3(s) + 3H2O(l) → Al(OH)3(s) + 3HCl (l)
- Ca3N2(s) + 6H2O(l) → 3Ca(OH)2(s) + 2NH3(g).
Question 21.
Describe the structure of common form of ice.
Answer:
1. Ice fleets over water. In ice (solid state), the H2O molecules are arranged tetrahedrally ini space. The oxygen atom in each H2O molecule is linked to two hydrogen atoms by covalent bonds and at the same time its forms hydrogen bonds with the hydrogen atoms of the neighbouring H2O molecules. This leads to a cage like structure as shown in the Figure 9.5. The structure is also porous because of the voids or empty spaces left.
Now, as ice mbits to form liquid water, the heat energy supplied tends to break some of the hydrogen bonds in H2O molecules. As a result, the tetrahedral arrangements start collapsing and the H2O molecules in water come closer to one another. The number of vacant spaces also start decreasing. Therefore, the density of water is more than that of ice. This means that ice always floats over water.
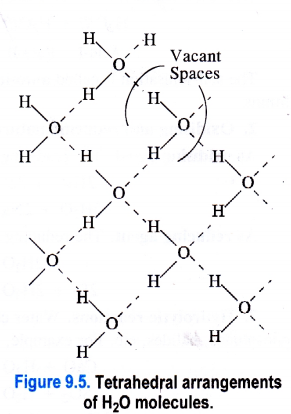
2. Water has maximum density at 4°C. At 273 K (or 0°C), both ice and water coexist. As the temperature is increased, the heat energy supplied will further break the tetrahedral arrangements because of decrease in hydrogen bonding. Therefore, the density of water is expected to increase. But the rise in temperature is also expected to increase the average kinetic energy of the H2O molecules leading to increase in volume (or “decrease in density). But this effect is negligible upto 4°C and the density of water, therefore, increases. However, if the temperature is increased beyond 4°C, then the effect of the increase in kinetic energy will be more as compared to the effect of increase in density. This is likely to increase the volume of water and decrease its density. Thus, we conclude that upto 4°C, the density of water increases and then decreases after this temperature. In other words, water has maximum density and minimum volume at 4°C.
This property of water is extremely helpful for the animals living under sea water. In severe cold, the surface of the sea almost freezes. But below the surface, there is water at a temperature of about 4°C. The aquatic animals can safely live in water at this temperature.
Question 22.
What causes temporary and permanent hardness of water ?
Answer:
1. Temporary hardness is due to the presence of hydrogen carbonates of calcium and magnesium dissolved in water. It is called temporary because it can be easily removed by simply boiling hard water.
2. Permanent hardness is due to the presence of chlorides and sulphates of calcium and magnesium dissolved in water. It is called permanent hardness as it cannot be removed on boiling hard water
Question 23.
Discuss the principle and method of softening of hard water by synthetic ion-exchange method.
Answer:
Softening of hard water. Softening of water means the removal of the ions from water which make it hard.
Question 24.
Write chemical reactions to show amphoteric nature of water.
Answer:
water covers nearly three-fourth of the earth’s surface in the form of snow over mountains as liquid in the rivers, lakes, springs and oceans. Next to oxygen, water is the most important for human life. One can live without food for a number of days but not without water. It is also used as a solvent for many substances and takes part in a variety of chemical reactions. Water is also a source of heavy water (D2O) which is extremely essential for nuclear reactors to control the speed of neutrons.
Question 25.
Hydrogen peroxide can act both as an oxidising as well as reducing agent. Explain.
Answer:
The Change in oxidation number accompanying the decomposition of H2O2 is as follows :
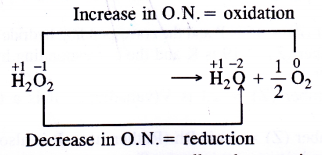
Since oxygen atom in H2O2 can undergo an increase as well as decrease in oxidation number therefore, it can act both as reducing as well as oxidising agent. This is supported by the following reactions.
As reducing agent:

In this reaction, H2O2 acts as reducing agent and has reduced Ag2O K to metallic Ag.
As oxidising agent:
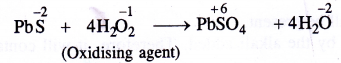
In this reaction, H2O2 has oxidised PbS to PbSO4.
Question 26.
What is meant by demineralised water ? How is it obtained ?
Answer:
Water free from cations (Ca2+, Mg2+ etc.) and anions (Cl–, SO–4, HCO3 etc.) responsible for hardness is known as de-ionised or demineralised water. It is formed by passing water repeatedly through cation and anion exchangers.
Now let us try to investigate as to why hard water does not form lather with soap readily. Actually soaps are water soluble sodium (or potassium salts) of higher fatty acids with formula NaX [where X may represent C17H45COO– (palmitate), C17H43COO– (oleate), or C17H35COO–(stereate) ions]. When soap is added to the hard water, the Na+ ions are replaced by Ca2+ and Mg2+ ions present in hard water to form the corresponding calcium and magnesium salts of the acids which get precipitated.

Thus, a lot of soap is wasted due to formation of curdy white precipitate and hard water is not useful in lauhdary. In addition, it cannot be employed in boilers for raising steam. Over a period of a time, the salts present in hard water get deposited inside the walls of the boiler in the form of hard scale. The boiler scale does not allow the flow of heat since it is a poor conductor. Sometimes, it also damages the boiler in case it cracks. Therefore, removal of these salts from water is necessary. This is called softening of water.
Question 27.
Is dimineralised or distilled water always useful for drinking purposes ? If not, how can it be made
useful ?
Answer:
No, dimineralised water or distilled water is not always useful for drinking purposes. It is usually tasteless. Moreover,some ions such as Na+, K+ and Mg2+ etc. are essential to the body. In order to make dimineralised water more useful, some useful salts of sodium and potassium etc. must be dissolved in it.
Question 28.
What properties of water make it useful as a solvent ? What type of compounds can it dissolve and hydrolyse ?
Answer:
Water is a useful, rather excellent solvent due to the following properties :
- It has high enthalpy of vaporisation and heat capacity.
- It is a liquid over a wide range of temperature (0° to 100°C)
- It is polar in nature and high dielectric constant (78-39).
It can dissolve polar substances and also some organic compounds due to hydrogen bonding.Water can hydrolyse oxides, halides, phosphides, nitrides etc.
Question 29.
Describe be the usefulness of water in biosphere and biological systems.
Answer:
- Water is a liquid with freezing point of 273-2 K and boiling point of 373-2 K.
- Water has the maximum density at 277 K (4°C) e. 1 gm cm-3.
- In water, the molecules are hydrogen bonded and the hydrogen bonding influences all the physical properties such as state, heat of fusion, heat of vaporisation, melting point and boiling point etc. For example, H2O is the hydride of the first member of group 16 e. oxygen. The rest of the members of the group are sulphur, selenium, tellurium and polonium. The hydrides of these elements do not show any hydrogen bonding and their physical properties are quite different from H2O. For example, H2O is a liquid while H2S is a gas at room temperature.
- Water is of polar nature. As a result, most of the inorganic substances and also many organic substances having polar bonds in their molecules are water soluble because df inter-molecular hydrogen bonding. In addition to this, water has high heat of vaporisation and it exists in the liquid form over a wide range of temperature (0 to 100°C). Therefore, water is regarded as an excellent solvent and is quite often knoy/n as universal solvent.
- Because of polar nature,’‘there are strong intermolecular forces of attraction and hydrogen bonding present in the H2O molecules. As a results water has higher specific heat, thermal conductivity, surface tension, dipole moment as compared to other liquids. ‘These properties enable water to play a major role in biosphere. It is responsible for maintaining the body temperature of living beings and also for moderating climate.
Question 30.
Knowing the properties of H2O and D2O, do you think that D2O can be used for drinking purposes ?
Answer:
Heavy water (D2O) is quite injurious to living beings, plants and animals since it slows down the rates of reactions which occur in them. It fails to supporfelife and has no utility in biosphere.
Question 31.
Hydrolysis is different from hydration. Elaborate.
Answer:
Hydrolysis is a chemical reaction in which a substance reacts with water under neutral, acidic or alkaline conditions. For example, aluminium chloride is hydrolysed by water as follows:
AlCl3 + 3H2O—>Al(OH)3 + 3HCl
Hydration on the other hand is the property of a chemical compound to take up molecules of water of crystallisation and get hydrated. For example, anhydrous copper sulphate (CuSO4) which is white in colour takes up five molecules of H2O to form hydrated copper sulphate (CuSO4 5H2O) which has blue colour.
![]()
Question 32.
Saline hydrides are generally used to remove traces of water from organic coin pounds. Explain.
Answer:
Saline hydrides (e.g., NaH, CaH2 etc.) absorb water and react with it as follows :
NaH(s) + H2O(l) → NaOH (ag) + H2(g)
CaU2(s) + 2H2O(l) →Ca(OH)2(aq) + H2(g)
The hydrpgen escapes leaving behind metal hydroxides. Thus, these hydrides can be used to remove traces of water from the organic compounds.
Question 33.
What do you expect the nature of hydrides if formed by the elements of atomic numbers 15, 19, 23 and 44 with dihydrogem?
Answer:
- The elefpent with atomic number (Z) = 15 is P and the corresponding hydride is phosphine [PH3]. It is covalent in nature.
- The element with atomic number (Z) = 19 is K and the corresponding hydride is potassium hydride (K+H“). It is ionic in nature.
- The element with atomic number (Z) = 23 is V(vanadium). It is a transition metal. It forms an interstitial hydride (VHx.g)
- The element with atomic number (Z) = 44 is Rh (Ruthenium). It is also a transition metal but does not form any hydride due to hydride gap (as it is present in group 8).Out of the hydrides listed, only the ionic hydrides react with water to evolve hydrogen gas.
2K+H-(s) + 2H2O(l)→ 2KOH(aq) + H2(g)
Question 34.
Do you are expect different products in solution when aluminum(III) chloride and potassium chloride are treated separately with (1) normal water (2) acidified water and (3) alkaline water ? (P.I.S.A. Based)
Answer:
Both the compounds are salts and they react in different manner with water of different nature.
(a) Aluminium (III) chloride or AlCl3 will react with water as follows :
AlCl3 + 3H2O →Al(OH)3 + 3HCl.
The reaction is known as hydrolysis.
- In normal water, Both Al(OH)3 and HCl will be present.
- In alkaline water, HCl will be neutralised by the alkali added. Therefore, it will contain mainly Al(OH)3 and will be alkaline in nature.
- In acidic water. In this medium, Al(OH)3 will be neutralised by the acid added. Therefore, water will contain mainly HCl and will be acidic in nature.
(b) Potassium chloride is a salt of strong acid and strong base. It will remain as such under all the conditions and will not undergo any chemical reaction.
Question 35.
How does H2O2 behave as a bleaching agent ?
Answer:
Bleaching nature of hydrogen peroxide is due to oxidation
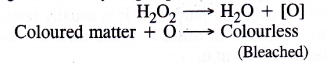
Question 36.
What do you understand by the terms :
(1) hydrogen economy
(2) hydrogenation
(3) syn gas
(4) water-gas shift reaction
(5) fuel cell ?
Answer:
1. Hydrogen Economy. The basic principle of hydrogen economy is the transportation and storage of energy in the form of liquid or gaseous dihydrogen. In India, dihydrogen was used for the first time in October 2005 for running automobiles like four- wheelers. Initially 5% dihydrogen was mixed with C.N.G. which is commonly used. The experiment is successful till to-day. Efforts are on to increase the percentage of dihydrogen gradually.
Hydrogen oxygen fuel cells can also be used to generate electricity to run electric powered cars. A fuel cell produces electricity from a chemical reaction just as in a battery and can work as long as hydrogen and oxygen are present. One such fuel cell made by German motors was on display at Sydney Olympics. A number of companies are working on these projects because of the limited availability and very high cost of petrol. A number of cars were launched in the year 2004 and it is predicted that by 2025, automobiles employing hydrogen as fuel will capture about 25% of the world market.
In these fuel cells, porous carbon electrodes are placed in a concentrated aqueous solution of KOH or NaOH. The temperature is maintained to about 400 K when the gases react to form water accompanied by the release of electrical energy. We will study in detail the working of fuel cells in next class under Electrochemistry .
2. Hydrogenation : Hydrogenation of vegetable oils. It is done by passing hydrogen gas through edible oils (groundnut oil, cotton seed oil etc.) in the presence of Ni at 473 K. As a result, the oils are converted into solid fats, also called vegetable ghees. Actually, oils are unsaturated due to the presence C=C bond. On hydrogenation, the bond changes into C—C bond and as a result, unsaturated oils change into saturated fats.
![]()
3.Syn gas : The mixture of CO and H2 was previously known as water gas. These days, it is known as synthesis gas or syn gas. In addition to hydrocarbons and coke, syn gas’ can also be produced from some other materials containing carbon and hydrogen, e.g., wood scrap, saw dust, newspapers, sewage etc. The process of preparing ‘syn gas’ from coke, coal and other materials is known as coal gasification.
4. Water-gas shift reaction :In place of iron chromate, a mixture of ferric oxide (Fe2O3) and chromium oxide (Cr2O3) can be used. This reaction is known as Water-gas shift reaction. From the gaseous mixture, carbon dioxide can be removed by either passing into water under pressure or by scrubbing with sodium arsenite solution.
5. Fuel cell : It is a cell which converts chemical energy of fuel directly into electrical energy.
We hope the NCERT Solutions for Class 11 Chemistry Chapter 9 Hydrogen, help you. If you have any query regarding NCERT Solutions for Class 11 Chemistry Chapter 9 Hydrogen, drop a comment below and we will get back to you at the earliest.