NCERT Class 12 Chemistry Solutions for Chapter 12 provides answers for the questions provided in the textbook. The answers are accurate to the best of our knowledge and are provided by subject experts. The students can refer to these or sure shot success.
The students appearing for various boards and competitive exams can find these solutions helpful for practice. Most of the questions asked in UP board, MP board, Gujarat board, CBSE, etc. are asked from these. To score well in the examinations, the students should go through these solutions atleast once after finishing the entire syllabus.
| Board | CBSE |
| Textbook | NCERT |
| Class | Class 12 |
| Subject | Chemistry |
| Chapter | Chapter 12 |
| Chapter Name | Aldehydes, Ketones and Carboxylic Acids |
| Number of Questions Solved | 28 |
| Category | NCERT Solutions |
NCERT Solutions for Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
This chapter explains the structure, physical and chemical properties and applications of aldehydes, ketones and carboxylic acid. It also explains a correlation between the three. It also explains the mechanism of different reactions of aldehydes, ketones and carboxylic acids. The factors affecting the acidity of carboxylic acids and their reactions help in understanding the advanced concepts related to this chapter.
NCERT Solutions for Class 12 Chapter 12 will help you revise the chapter during the examination. It also helps in clearing doubts if any.
NCERT INTEXT QUESTIONS
Question 1:
Write the structures of the following compoimds :
(i) α -Methoxypropionaldehyde
(ii) 3-Hydroxybutanal
(iii) 2-Hydroxycyclopentanecarbaldehyde
(iv) 4-Oxopentanal
(v) Di-sec butylketone
(vi) 4-Fluoroacetophenone
Answer:
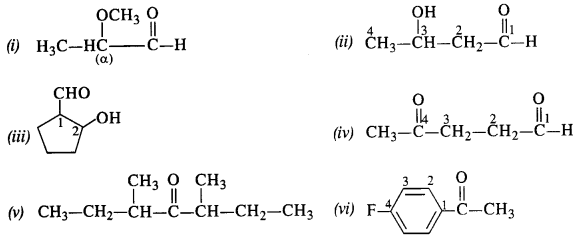
Question 2.
Write the structures of products of following reactions:
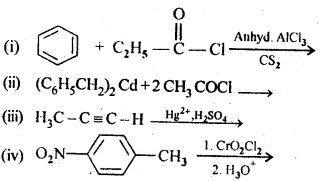
Answer:
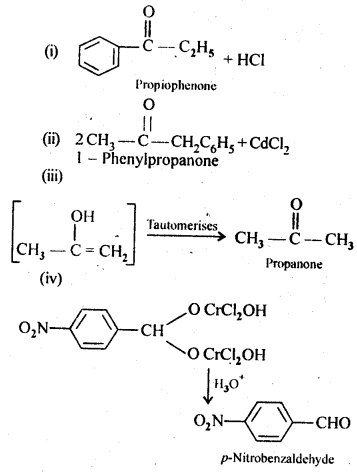
Question 3.
Arrange the following compounds in increasing order of their boiling points :
CH3CHO, CH3CH2OH, CH3OCH3, CH3CH2CH3
Answer:
The increasing order of boiling points of all these compounds of comparable molecular masses is :
![]()
Explanation: We know that the boiling points of liquids are directly related to the magnitude of the intermolecular forces of attraction.
- Hydrocarbons (alkanes) are completely non-polar. The only attractive forces in their molecules are Van der Waals forces which are quite weak. That is why propane (CH3CH2CH3) has the least boiling point. It is a gas at room temperature.
- Ethers have bent structures and are also polar. However, there is no hydrogen bonding in their molecules. The only attractive forces are dipolar forces. Therefore, boiling point of dimethyl ether (CH3OCH3) is higher than that of propane. However, it is also a gas at room temperature.
- Aldehydes contain polar carbonyl group and have strong dipolar interactions in their molecules. It is more than in ethers. Therefore, the boiling point of acetaldehyde (CH3CHO) is more than that of dimethyl ether.
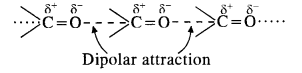
- Out of all the families listed, alcohols have maximum intermolecular forces in the form of hydrogen bonding
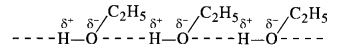
As a result, ethyl alcohol (C2H5OH) has the maximum boiling point.
Question 4.
Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions
(i) Ehtanal, propanaL, propanone, butanone
(ii) Benzaldehyde,p-Tolualdehyde, p-Nitrobenzaldehyde, acetophenone.
Ans: (i) Butanone < Propanone < Propanal < Ethanal .This is because as the no. of alkyl groups attached to carbonyl carbon increases, +I-effect increases. As a result, e– density
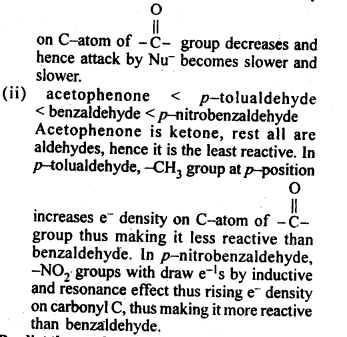
Question 5.
Predict the products of the following reactions :
Answer:
Question 6.
Give the IUPAC names of the following compounds:

Answer:


Question 7.
Show how each of the following compounds can be converted into benzoic acid?
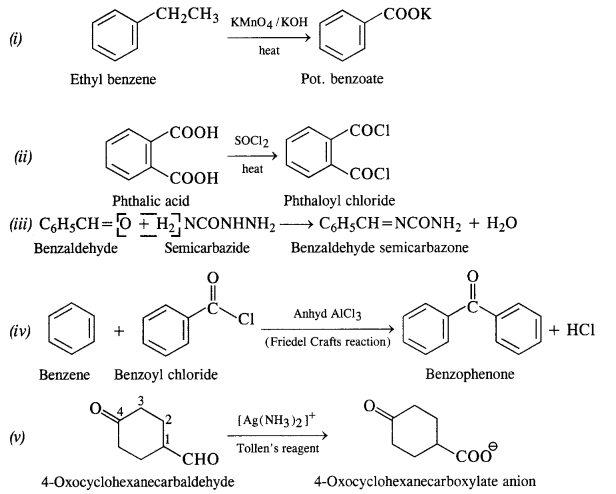
(i) Ethylbenzene (C.B.S.E. Outside Delhi 2017)
(ii) Acetophenone (C.B.S.E. Outside Delhi 2017)
(iii) Bromobenzene
(iv) Phenylethene (Styrene)
Answer:
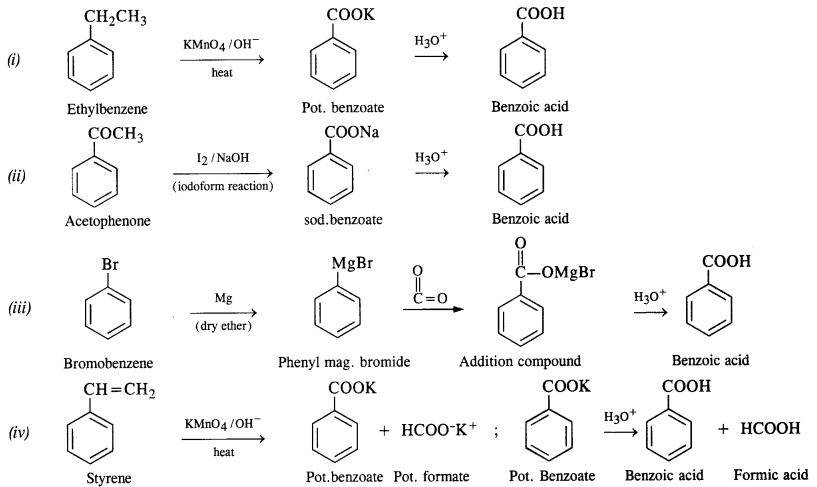
Question 8.
Which acid of each pair would you expect to be stronger?
(i) CH3CO2H or FCH2CO2H
(ii) FCH2CO2H or ClCH2CO2H
(iii) FCH2CH2CH2CO2H or CH3CH(F)CH2CO2H
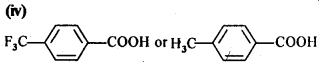
Answer:
NCERT EXERCISE
Question 1.
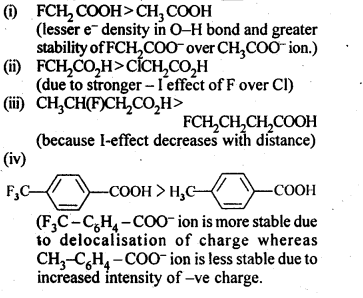
What is meant by the following terms? Give an example in each case.
(a) Cyanohydrin
(b) Semicarbazone
(c) Acetal
(d) Oxime
(e) Cyanohydrin
(f) Ketal
(g) Aldol
(h) Schiff’s base
(i) 2, 4-D.N.P.
(ii) Imine
Answer:
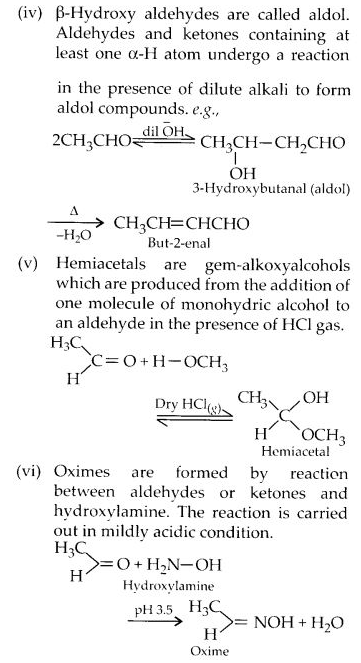
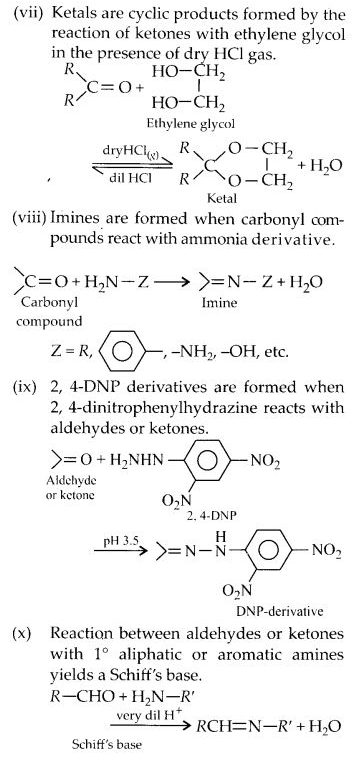
Question 2.
Name the following compounds according to IUPAC system of nomenclature.
(i) CH3CH(CH3)CH2CH2CHO
(ii) CH3CH=CHCHO
(iii) CH3CH(CH3)CH2C(CH3)2COCH3
(iv) OHCC6H4CHO(-p)
(v) CH3CH2COCH(C2H5)CH2CH2Cl
(vi) CH3COCH2COCH3
(vii) (CH3)3CCH2COOH
(viii) (CH3)2CHCH(CH3)COCl
Answer:
(i) 4-Methylpentanal
(ii) But-2-enal
(iii) 3 3 5-Trimethylhexan-2-one
(iv) Benzene- 1, 4-dicarbaldehyde
(v) 6-Chloro-4-ethylhexan-3-one
(vi) Pentane-2, 4-dione
(vii) 3, 3-Dimethylbutanoic acid
(viii) 2, 3-Dimethylbutanoyl chloride
Question 3.
Draw the structures of the following compounds:
(i) 3-Methylbutanal
(ii) p-Nitropropiophenone
(iii) p-Methylbenzaldehyde
(iv) 4-Methylpent-3-en-2-one
(v) 4-Chloropentan-2-one
(vi) 3-Bromo-4-phenylpentanoic acid
(vii) pp’-Dihydroxybenzophenone
(viii) Hex-2-en-4-ynoic acid
Answer:
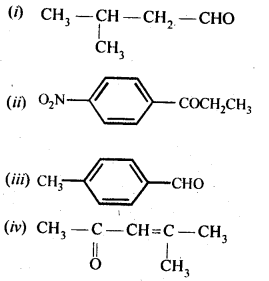
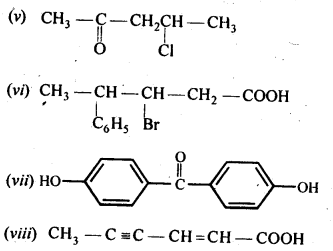
Question 4.
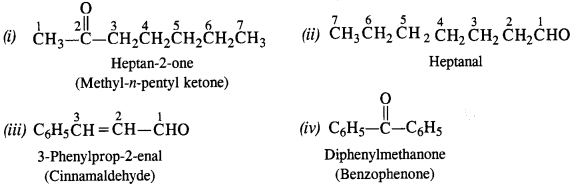
Write the IUPAC names of the following aldehydes and ketones. Also give the common names wherever possible.

Answer:
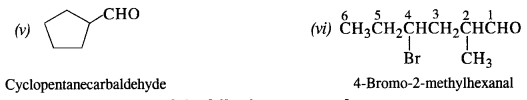
Draw the structures of the following compounds :
(a) 2, 4-dinitrophenylhydrazone of benzaldehyde
(b) Cyclopropanone oxime
(c) Acetaldehyde dimethyl acetal
(d) Semicarbazone of cyclobutanone
(e) Ethylene ketal of hexan-3-one
(f) Methyl hemiacetal of formaldehyde.
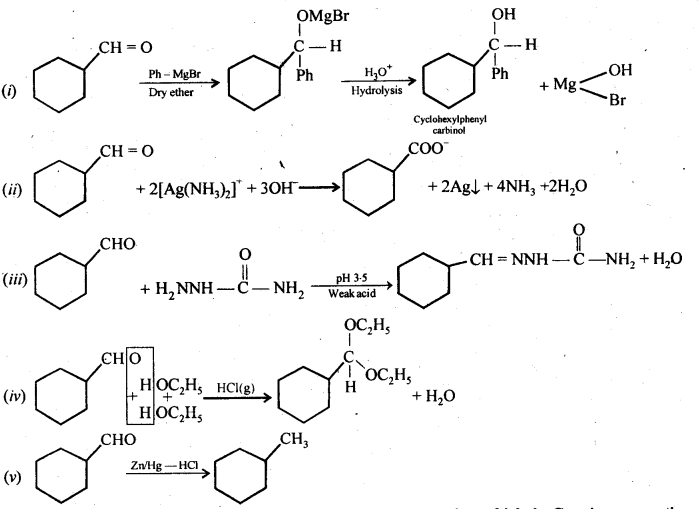
Question 6.
Predict the products formed when cyclohexane carbaldehyde reacts with the following reagents.
(i) PhMgBr and then H3O+
(ii) Tollen reagent
(iii) Semicarbazide and weak acid
(iv) Excess ethanol and acid
(v) Zinc amalgam and dilute hydrochloric acid
Answer:
Question 7.
Which of the following will undergo Aldol condensation, which Cannizzaro’s reaction, and which neither of these? Write the structures of the expected products in each case
(i) Methanal
(ii) 2-Methylpentanal
(iii) Benzaldehyde
(iv) Benzophenone
(v) Cyclohexanone
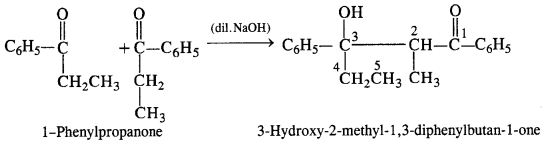
(vi) 1-Phenylpropanone
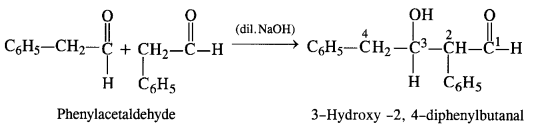
(vii) Phenylacetaldehyde
(viii) Butan-1-ol
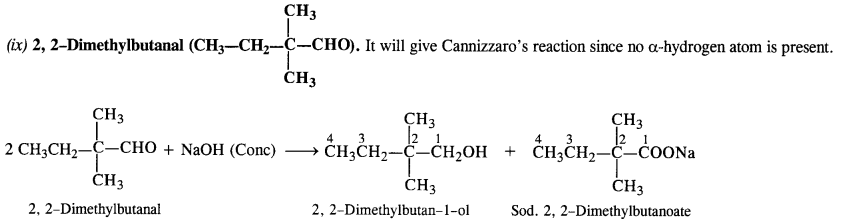
(ix) 2, 2-Dimethyl butanal
Answer:
(i) Methanal (HCHO): It will give Cannizzaro’s reaction since the α-hydrogen atom is absent.
![]()
(ii) 2-Methylpentanal [CH3CH2CH2CH (CH3)CHO]: It will give Aldol condensation since the α-hydrogen atom is present.
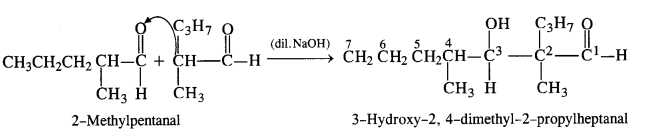
(iii) Benzaldehyde (CgH5CHO): It will give Cannizzaro’s reaction since a-hydrogen is not present.
![]()
(iv) Benzophenone (C6H5COC6H5): It will not give any of the two reactions. Being ketone, does not take part in Cannizzaro’s reaction. Without a-hydrogen, it fails to participate in Aldol condensation.
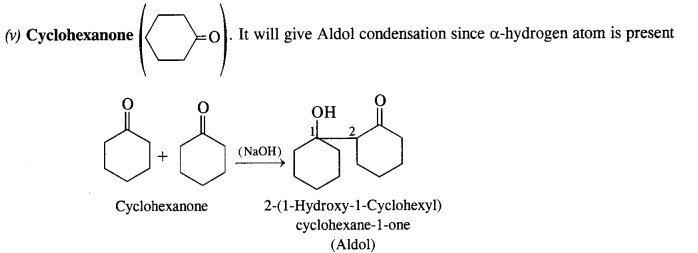
(vi) 1-Phenylpropanone (C6H5COCH2CH3): It will undergo Aldol condensation since the α-hydrogen atom is present.
(vii) Phenylacetaldehyde (C6H5CH2CHO): It will give Aldol condensation since the α-hydrogen atom is present.
(viii) Butan-1-ol: It will not give any of the reactions.
Question 8.
How will you convert ethanal to the following compounds?
(i) Butane-1, 3-diol
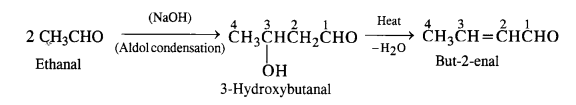
(ii) But-2-enal
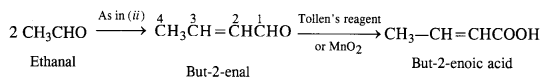
(iii) But-2-enoic acid.
Answer:
(i) Ethanal to butane -1, 3-diol
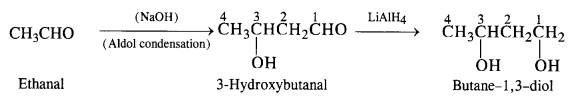
(ii) Ethanal to but-2-enal
(iii) Ethanal into but-2-enoic acid
Question 9.
Write the structural formulae and names of four possible aldol condensation products from propanal and butanal. In each case, indicate which aldehyde serves as a nucleophile and which as an electrophile.
Answer:
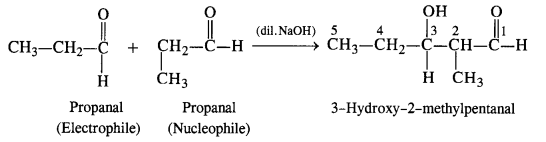
Both propanal and butanal have a-hydrogen atoms present. These can undergo self aldol condensation as well as cross aldol condensation to give four compounds as follows:
(i) Condensation involving propanal: It is a case of a self aldol condensation.
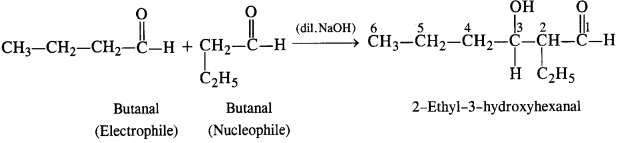
(ii) Condensation involving butanal: It is self aldol condensation.
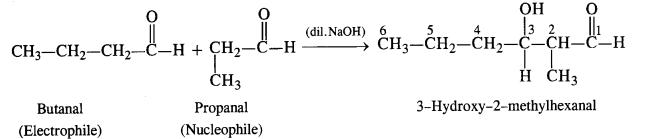
(iii) Condensation involving butanal (electrophile) and propanal (nucleophile): It is cross-aldol condensation.
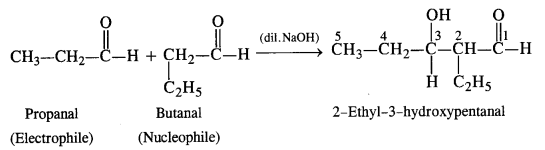
(iv) Condensation involving propanal (electrophile) and butanal (nucleophile): It is cross-aldol condensation.
Question 10.
An organic compound with molecular formula CgHjoO forms 2, 4-DNP derivative, reduces Tollen’s reagent, and undergoes Cannizzaro’s reaction. On vigorous oxidation, it gives 1, 2 benzene dicarboxylic acid. Identify the compound. (C.B.S.E. Outside Delhi 2012; Haryana Board 2013)
Answer:
Since the compound forms 2, 4-DNP derivative on reacting with 2, 4-DNP, it is a carbonyl compound. As the compound reduces Tollen’s reagent and undergoes Cannizzaro’s reaction, it is an aldehyde and not a ketone. The data further reveals that the compound on vigorous oxidation gives 1, 2-benzene dicarboxylic acid. This clearly shows that in the compound which is of aromatic nature, CHO group is present at position-1 and C2H5 side chain at position-2. The given compound is 2-ethyl benzaldehyde. The reactions involved are given below :
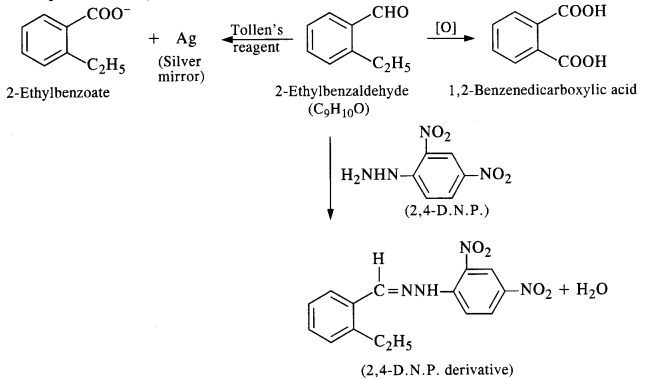
Question 11.
An organic compound [A] with molecular formula C8H16O2 was hydrolysed with dilute sulphuric acid to give a carboxylic acid [B] and alcohol [C]. Oxidation of [C] with chromic acid produced [B]. The alcohol [C] on dehydration gave but-1-ene. Write equations for the reactions involved. (C.B.S.E. 2008 Supp., C.B.S.E. 2009)
Answer:
(i) The available data shows that the compound [A] upon hydrolysis gave a carboxylic acid [B] and alcohol [C]. It must be an ester.
(ii) Since the alcohol [C] upon oxidation with chromic acid gave back the carboxylic acid [B], both the acid and alcohol must have the same number of carbon atoms (four each).
(iii) The alcohol [C] upon dehydration gave an alkene.
The equations for the reactions are given:
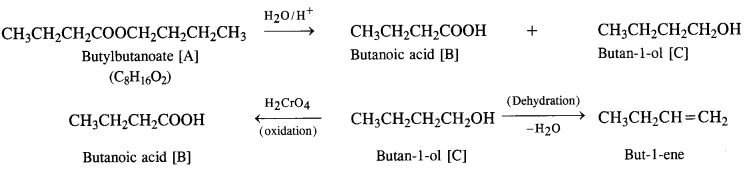
Question 12.
Arrange the following compounds in increasing order of their property as indicated:
(i) Acetaldehyde, Acetone, Di-tert butyl ketone, Methyl tert-butyl ketone (reactivity towards HCN)
(ii) CH3CH2CH(Br)COOH, CH3CH(Br)CH2COOH, (CH3)2CH COOH, CH3CH2CH2COOH (acid strength).
(iii) Benzoic acid, 4-Nitrobenzoic acid, 3,4-Dinitrobenzoic add, 4-Methoxybenzok acid (acid strength).
Answer:
(i) The reactivity of aldehydes and ketones towards HCN addition decreases as the +1 – effect of the alkyl groups increases. Secondly, it decreases with increase in steric hindrance to the nucleophilic attack by CN– at the carbonyl carbon. Thus the decreasing order of reactivity towards HCN is,
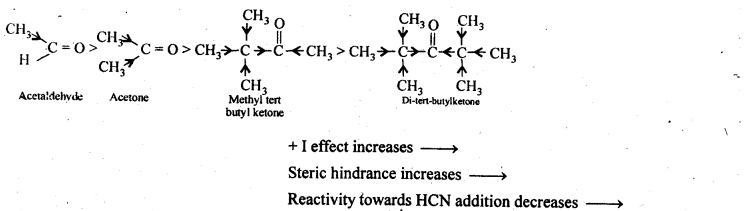
(ii) We know that the + I-effect decreases while -I-effect increases the acidic strength of carboxylic acids. Since + I-effect of isopropyl group is more than that of propyl group, therefore, (CH3)2CHCOOH is a weaker acid than CH3CH2CH2COOH. Further since -I-effect decreases with distance, therefore CH3CH2CHBrCOOH is a stronger acid than CH3CHBrCH2COOH. Thus, the overall acid strength increases in the order:
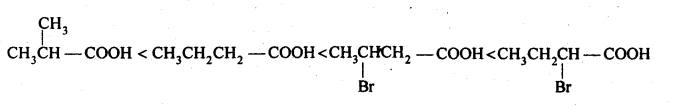
(iii) Since electron-donating groups decrease the acidic strength, therefore, 4-methoxy benzoic acid is a weaker acid than benzoic acid. Further, since electron-withdrawing groups increase the acidic strength, therefore, both 4-nitrobenzoic acid and 3,4-dinitrobenzoic acid are stronger acids than benzoic acid. Further due to the presence of an additional -NO2 group at /w-position with respect to -COOH group, 3,4-dinitrobenzoic acid is a stronger acid than 4-nitrobenzoic acid. Thus, the overall acidic strength increases in the order:4-methoxy benzoic acid < benzoic acid < 4-nitrobenzoic acid < 3,4-dinitrobenzoic acid.
Question 13.
Give chemical tests to distinguish between the following pairs of compounds :
(i) Propanal and propanone (C.B.S.E. Delhi 2011, 2012)
(ii) Phenol and benzoic acid
(iii) Acetophenone and benzophenone
(iv) Benzoic acid and ethyl benzoate (C.B.S.E. Outside Delhi 2009, 2011)
(v) Pentan-2-one and pentane-3-one
(vi) Benzaldehyde and acetophenone (C.B.S.E. Outside Delhi 2015)
(vii) Ethanal and propanal (C.B.S.E. Outside Delhi 2009, 2011, 2012)
Answer:
(i) Propanal and propanone: Propanal will give a silver mirror upon heating with Tollen’s reagent but propanone will not respond.
(ii) Phenol and benzoic acid: Benzoic acid will give brisk effervescence with sodium hydrogen carbonate (NaHC03) but phenol will not respond.
(iii) Acetophenone and benzophenone: Acetophenone is a methyl ketone. It will give a yellow precipitate upon heating with I2 and NaOH. Benzophenone will not respond.
(iv) Benzoic acid and ethyl benzoate: Benzoic acid will give brisk effervescence with sodium hydrogen carbonate (NaHC03) but ethyl benzoate (ester) will not respond.
(v) Pentan-2-one and pentan-3-one: Pentan-3-one is a methyl ketone and will give a yellow precipitate upon heating with I2 and NaOH. Pentan-3-one will not respond.
(vi) Benzaldehyde and acetophenone: The distinction can also be made by iodoform test. Acetophenone will give yellow precipitate while benzaldehyde will not react.
(vii) Ethanal and propanal: Ethanal will respond to iodoform test and give yellow precipitate. Propanal will not react.
Question 14.
How will you prepare the following compounds from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom.
(i) Methylbenzoate
(ii) m-Nitrobenzoic acid
(iii) p-Nitrobenzoic acid
(iv) Phenylacetic acid
(v) p-nitrobenzaldehyde
Answer:
(i) Benzene to methylbenzoate
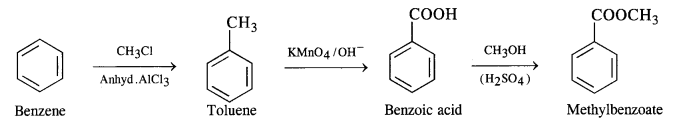
(ii) Benzene to m-nitrobenzoic acid
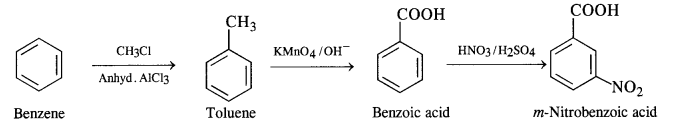
(iii) Benzene to p-nitrobenzoic acid
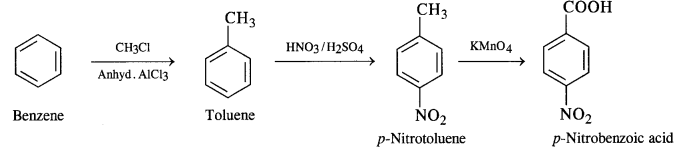
(vi) Benzene to phenylacetic acid
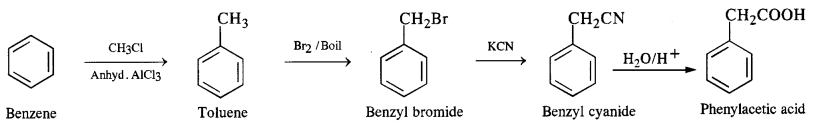
(v) Benzene to p-nitrobenzaldehyde
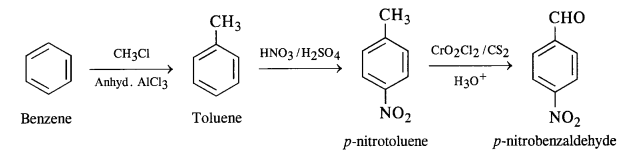
Question 15.
How will you bring about the following conversions in not more than two steps?
(i) Propanone to propene (C.B.S.E. Delhi 2009, Uttarakhand Board 2009)
(ii) Propanal to butanone
(iii) Ethanol to 3-hydroxybutanal (C.B.S.E. Outside Delhi 2012)
(iv) Benzaldehyde to benzophenone (C.B.S.E. Outside Delhi 2012)
(v) Benzaldehyde to 3-PhenyIpropan-1-ol
(vi) Benzaldehyde to a-Hydroxyphenylacetic acid
(vii) Benzoic acid to benzaldehyde (C.B.S.E. Delhi 2009, Outside Delhi 2017)
(viii) Benzene to m-nitroacetophenone
(ix) Benzoic acid to /n-nitrobenzyl alcohol. (C.B.S.E. Delhi 2012)
Answer:
(i) Propanone to propene
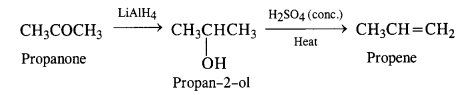
(ii) Propanal to butanone
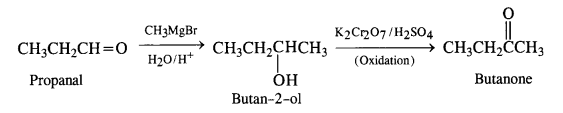
(iii) Ethanol to 3-hydroxybutanal
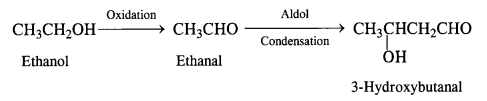
(iv) Benzaldehyde to benzophenone
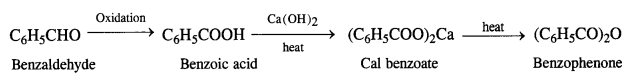
(v) Benzaldehyde to 3-phenylpropan-1-ol
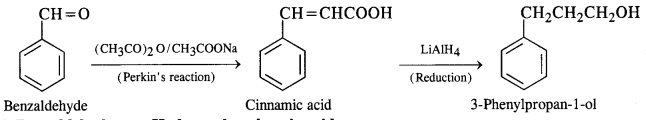
(vi) Benzaldehyde to a-Hydroxyphenylacetic acid
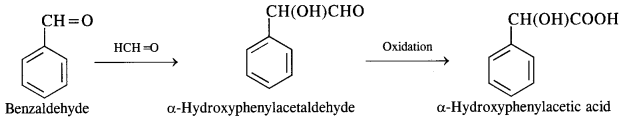
(vii) Benzoic acid to benzaldehyde
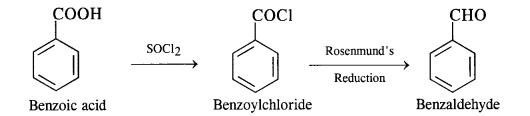
(viii) Benzene to m-nitroacetophenone
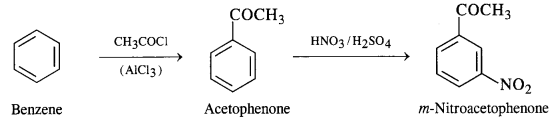
(ix) Benzoic acid to m-nitrobenzyl alcohol
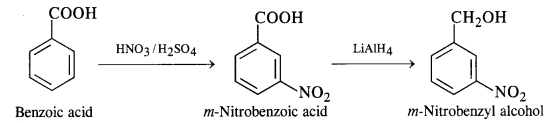
Question 16:
Describe the following:
(i) Acylation
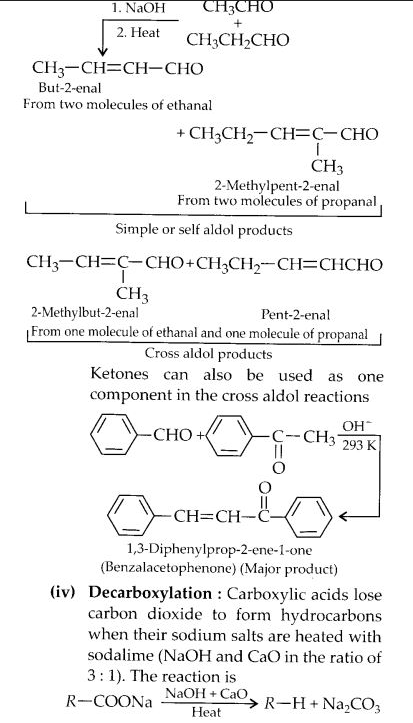
(ii) Cross-aldol condensation
(iii) Cannizzaro’s reaction
(iv) Decarboxylation.
Answer:
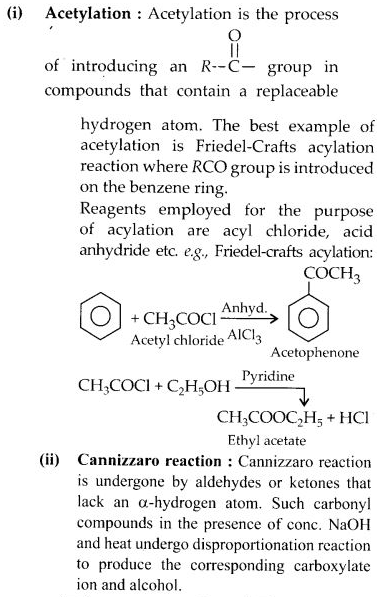
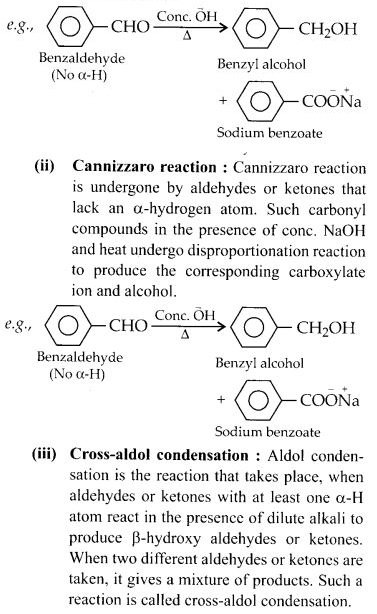
Question 17:
Complete each synthesis by giving missing starting material, reagent, or products.
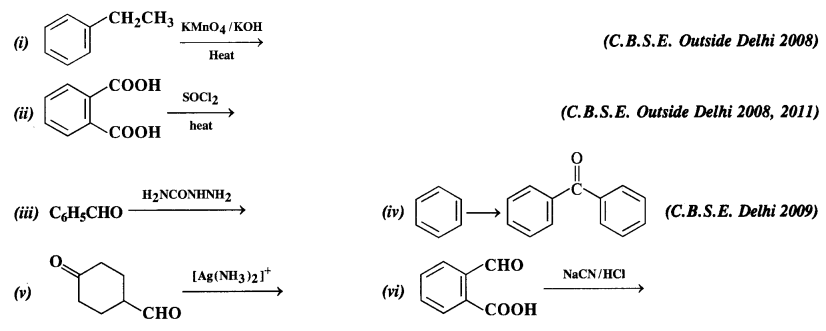
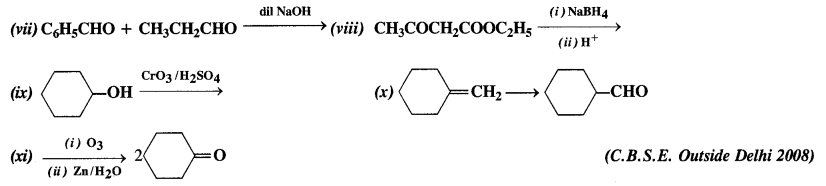
Answer:
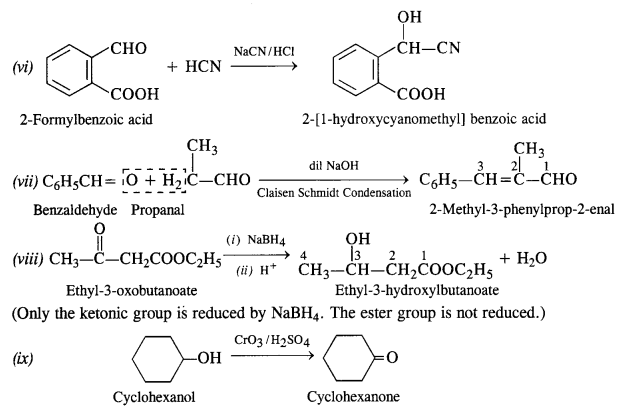
Question 18:
Give a plausible explanation for each of the following:
(i) Cyclohexanone forms cyanohydrin in good yield but 2, 4, 6-trimethylcyclohexanone does not.
(ii) There are two – NH2 groups in semicarbazide. However, only one is involved in the formation of semicarbazone.
(iii) During the preparation of esters from carboxylic acid and alcohol in the presence of an acid catalyst, the water or the ester should be removed as fast as it is formed.
Answer:
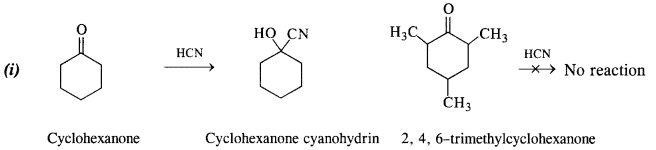
In cyclohexanone, the attack of CN“ ion (nucleophile) can easily take place at the carbonyl carbon atom. However, in 2, 4, 6-trimethylcyclohexanone, the three CH3 groups being electron releasing in nature (+ I effect) will considerably increase the electron density on the carbonyl carbon atom and the nucleophile attack does not seem to be feasible. Moreover, the two —CH3 substituents at the ortho positions will also hinder the attack of nucleophile CN– ion on the carbonyl group.
(ii) The structural formula of semi-carbazide is NH2NHCONH2. Although both the amino groups have lone electron pairs, one of these is in conjugation with the electron-withdrawing carbonyl group and acquires a positive charge. Therefore, it is not in a position to act as the nucleophile, and only one -NH2 group is involved in the formation of semicarbazone.
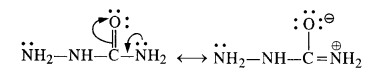
(iii) The esterification carried in the presence of acid is of reversible nature and the reverse reaction is called ester hydrolysis.
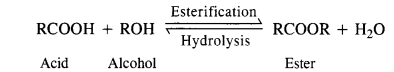
In order that the reaction may proceed in the forward direction, ester or water formed in the reaction must be removed. Sulphuric acid added in esterification helps in removing molecules of H20 as it is a dehydrating agent.
Question 19:
An organic compound contains 69-77% carbon, 11-63% hydrogen and the rest is oxygen. The molecular mass of the compound is 86. It does not reduce Tollen’s reagent but forms an addition compound with sodium hydrogen sulphite and gives a positive iodoform test. On vigorous oxidation, it gives ethanoic acid and propanoic acid. Write the possible structure of the compound. (C.B.S.E. Delhi 2008, 2009, Uttarakhand Board 2015)
Answer:
Step I: Calculation of molecular formula of the compound
Percentage of oxygen = 100 – (% C + % H) = 100 – (69.77 + 11.63) = 18.6%
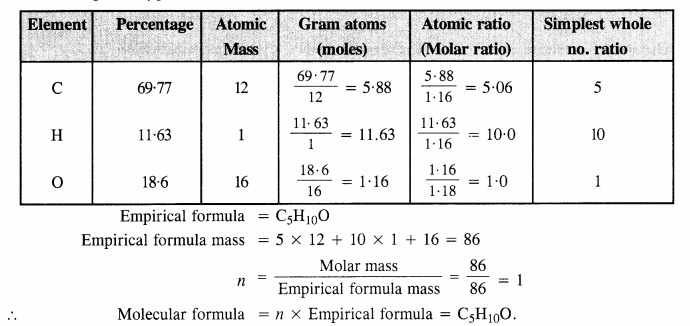
Step II. Predicting the structure of the compound
- Since the compound forms an addition compound with NaHS03, it must be a carbonyl compound.
- As the compound does not reduce Tollen’s reagent but gives a positive iodoform test, it must contain in it a methyl
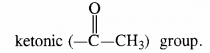
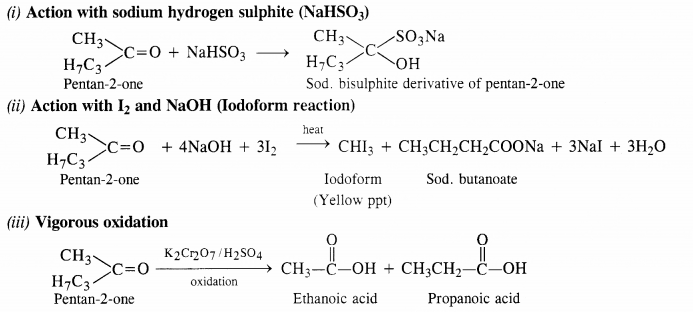
Keeping in view these characteristics, the compound is CH3CH2CH2COCH3 (Pentan-2-one).
All the reactions in which pentan-2-one participates, are given for the benefit of the students.
Question 20.
Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid than phenol. Why?
Answer:
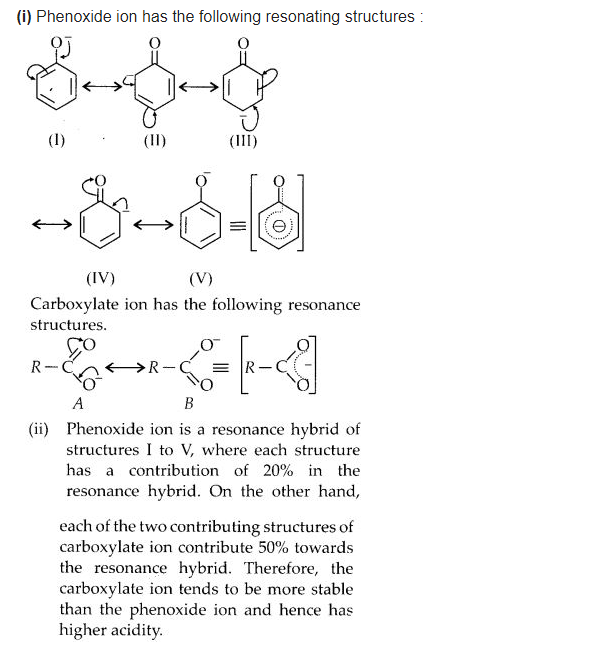

We hope the NCERT Solutions for Class 12 Chemistry Chapter 12 Aldehydes, Ketones, and Carboxylic Acids help you. If you have any query regarding NCERT Solutions for Class 12 Chemistry Chapter 12 Aldehydes, Ketones, and Carboxylic Acids, drop a comment below and we will get back to you at the earliest.