Class 12 NCERT Solutions for Chemistry Chapter 13 contains solved answers for the questions provided in the textbook. These answers are provided by the subject experts and are accurate to the best of our knowledge. The students can use these answers along with the diagrammatic representation in the examination and score well.
NCERT Solutions are beneficial for the students appearing for UP board, MP Board, CBSE, Gujarat board, etc., and also for competitive exams such as NEET and JEE.
| Board | CBSE |
| Textbook | NCERT |
| Class | Class 12 |
| Subject | Chemistry |
| Chapter | Chapter 13 |
| Chapter Name | Amines |
| Number of Questions Solved | 23 |
| Category | NCERT Solutions |
NCERT Solutions for Class 12 Chemistry Chapter 13 Amines
This chapter explains the importance, structure, physical and chemical properties, and the methods of preparation of amines. This chapter also explains the diazonium salts and the methods of their preparation. This will help you to understand the importance of these salts in the synthesis of aromatic compounds.
The students can use NCERT Solutions for better preparations of the concepts mentioned in this chapter. Basic understanding is very important for advanced concepts.
NCERT INTEXT QUESTIONS
Question 1.
Classify the following amines as primary, secondary, and tertiary amines
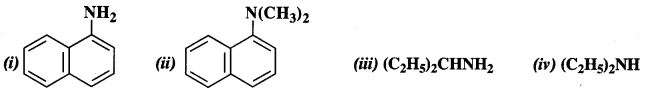
Answer:
(i) primary
(ii) tertiary
(iii) primary
(iv) secondary.
Question 3.
(i)Write the structures of different isomeric amines corresponding to the molecular formula, C4H11N.
(ii) Write 1UPAC names of all the isomers.
(iii) What type of isomerism is exhibited by different pairs of amines?
Answer:
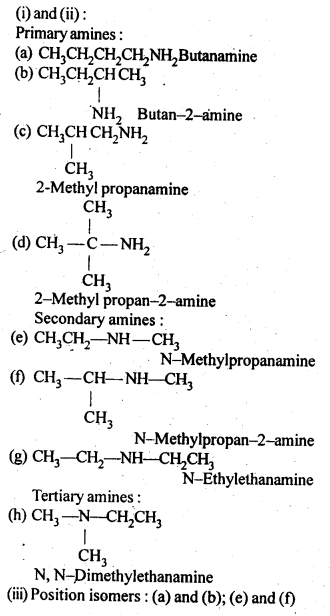
Chain isomers: (a) and (c); (a) and (d); (b) and (c); (b) and (d)
Metamers: (e) and (g); (f) and (g)
Functional isomers: All 10 amines are functional isomers of 2° and 3° amines and vice-versa.
Question 3.
How will you convert:
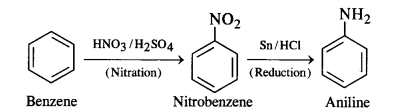
(i) Benzene into aniline
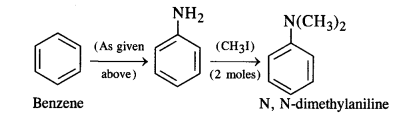
(ii) Benzene into N, N-dimethylaniline
(iii) C1(CH2)6Cl into hexane-1, 6-diamine
Answer:
(i) Benzene into aniline:
(ii) Benzene into N, N-dimethylaniline:
(iii) Cl(CH2)6Cl into hexane-1, 6-diamine:
![]()
Question 4.
Arrange the following in increasing order of their basic strength:
(i) C2H5NH2,C6H5NH2,NH3,C6H5CH2NH2 and(C2H5)2 NH
(ii) C2H5NH2,(C2H5)2NH,(C2H5)3N,C6H5NH2
(iii) CH3NH2, (CH3)2NH, (CH3)3N, C6H5NH2, C6H5CH2NH2
Answer:
(i) C6H5NH2 < NH3 < C6H5CH2NH2 < C2H5NH2<(C2H5)2NH
(ii) C6H5NH2 < C2H5NH2 < (C2H5)3N <(C2H5)2NH
(iii) C6H5NH2 < C6H5CH2NH2 < (CH3)3 N < CH3NH2<(CH3)2NH
Question 5.
Complete the following acid-base reactions and name the products
(i) CH3CH2CH2NH2 + HC1 →
(ii) (C2H5)3N + HC1 →
Answer:
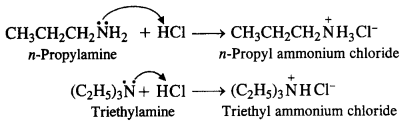
Question 6.
Write the reactions of the final alkylation product of aniline with excess methyl iodide in the presence of sodium carbonate solution.
Answer:
Aniline is a primary amine. It will react with excess methyl iodide to form quaternary ammonium salt as the final product. The reaction is known as Hoffmann’s ammonolysis.
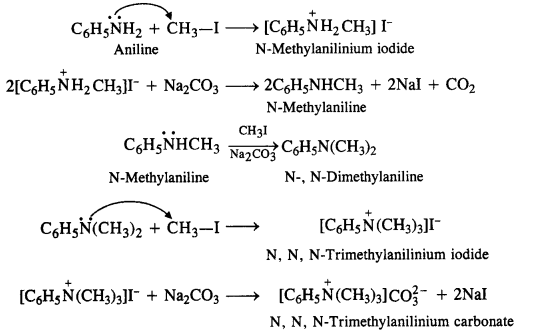
Question 7.
Write the chemical reaction of aniline with benzoyl chloride and write the name of the product obtained.
Answer:
Aniline will undergo benzoylation to form a benzoyl derivative. The reaction will take place in the presence of aqueous alkali.
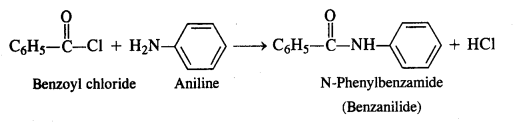
Question 8.
Write the structures of the different isomers corresponding to the molecular formula C3H9N. Write IUPAC names of the isomers which will liberate nitrogen gas on treatment with nitrous acid.
Answer:
Four isomeric aliphatic amines are represented by the molecular formula C3H9N. These are:
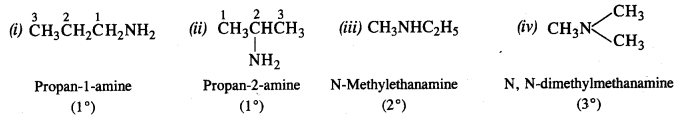
Only the primary amines will evolve N2 gas on reacting with nitrous acid (HONO) and form corresponding primary alcohols.
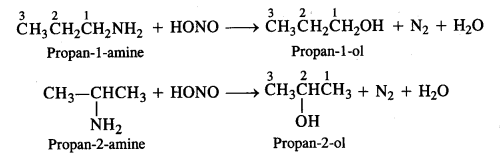
Question 9.
How will you convert:
(i) 3-Methylaniline into 3-nitrotoluene
(ii) Aniline into 1,3,5-tribromobenzene ?
Answer:
(i) 3-Methylaniline into 3-nitrotoluene
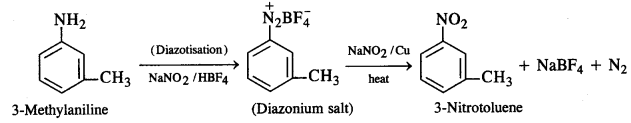
(ii) Aniline into 1,3,5-tribromobenzene
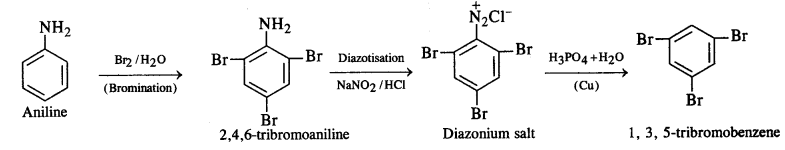
NCERT EXERCISE
Question 1.
Write IUPAC names of the following compounds and classify them into primary, secondary and tertiary amines.
(i) (CH3)2CHNH2
(ii) CH3(CH2)2NH2
(iii) CH3NHCH(CH3)2
(iv) (CH3)3CNH2
(v) C6H5NHCH3
(vi) (CH3CH2)2NCN3
(vii) m-BrC6H4NH2
Answer:
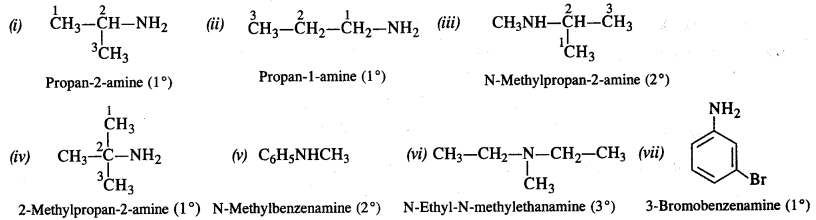
Question 2.
Give one chemical test to distinguish between the following pairs of compounds :
(i) Methylamine and dimethylamine
(ii) Secondary and tertiary amines
(iii) Ethylamine and aniline
(iv) Aniline and benzylamine
(v) Aniline and N-methylaniline. (C.B.S.E. Sample Paper 2015)
Answer:
(i) Methylamine on reaction with nitrous acid evolves N2 gas with brisk effervescence while dimethylamine does not. Methylamine also gives carbylamine reaction upon warming with chloroform and alcoholic KOH while dimethylamine does not.
(ii) Secondary amines, both aliphatic and aromatic respond to Libermann’s nitroso reaction while tertiary amines do not.
(iii) Aniline responds to diazotisation and coupling reactions to form a dye while ethylamine does not.
(iv) Aniline gives diazotisation coupling reaction while benzylamine does not.
(v) Aniline gives carbyl amine test with an extremely unpleasant smell while N-Methyl aniline does not.
Question 3.
Account for the following :
(i) pKb of aniline is more than that of methylamine. (C.B.S.E. Delhi 2008, 2011)
(ii) Ethylamine is soluble in water whereas aniline is not. (C.B.S.E. Delhi 2008, 2011)
(iii) Methylamine in water reacts with ferric chloride to precipitate hydrated ferric oxide. (C.B.S.E. Delhi 2008)
(iv) Although the amino group is o- and p-directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline. (C.B.S.E. Sample Paper 2010)
(v) Aniline does not undergo Friedel Crafts reaction. (C.B.S.E. Delhi 2008, Sample Paper 2010, C.B.S.E. Outside Delhi 2015)
(vi) Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
(vii) Gabriel phthalimide synthesis is preferred for synthesising primary amines.
Answer:
(i) pKb of aniline is more than that of methylamine because aniline is less basic. In aniline, the electron pair on the nitrogen atom is involved in conjugation with the ring and is less available for protonation than in methylamine. Therefore, aniline has more pKb.
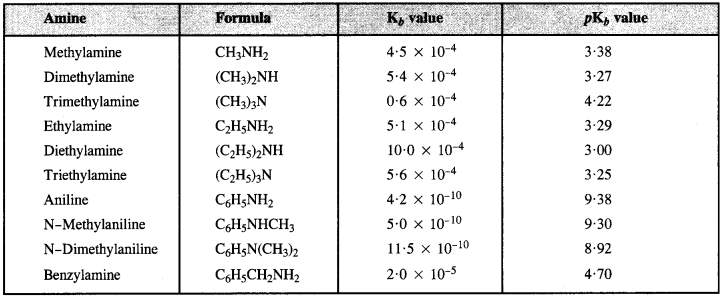
(ii) Ethylamine is water-soluble due to hydrogen bonding. However, in aniline, the phenyl (C6H5) group is bulky in size and has -I effect. As a result, its hydrogen bonding with water is negligible and is therefore not soluble or miscible with water.
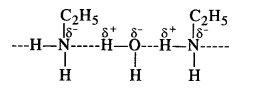
(iii) Methylamine forms a soluble hydroxide on reacting with water. The OH– ions released by the hydroxide combine with Fe3+ ions of ferric chloride to give ferric hydroxide or hydrated ferric oxide which is brown in colour.
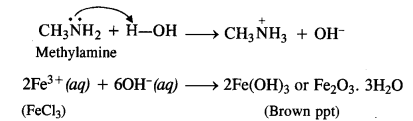
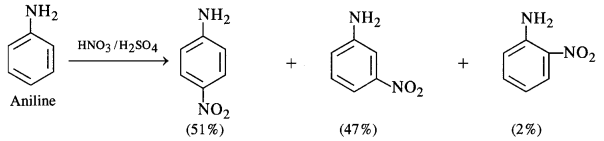
(iv) Amino group (-NH2) is an electron releasing or activating group when present on the benzene ring. It activates the ortho and para positions in the ring towards electrophilic substitution due to its +M or +R effect. The nitration of aniline carried by nitrating mixture (cone. HN03 + cone. H2SO4) is electrophilic in nature. The expected product of nitration is a mixture of ortho and para nitroaniline. However, in this case, a substantial amount of metanitroaniline is also formed. In fact, aniline being a base gets protonated in the acidic medium to form anilinium cation which is no longer activating. Rather, it is deactivating in nature and deactivates the ring. The substitution takes place at the meta position.
Thus, the nitration of aniline as such gives a significant amount of m-nitroaniline (47%). In addition to this, p-nitroaniline
is the major constituent (51%) while ortho isomer is in negligible amount (2%) mainly due to the reason that the ortho position
is sterically hindered because of the —NH2 group.
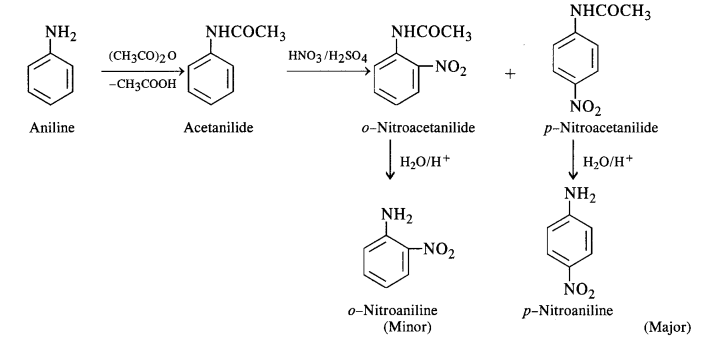
In order to check the activation of the ring by an amino group, the nitration of aniline is carried out indirectly by first
acetylating with acetic anhydride (or acetyl chloride) to form acetanilide. The compound formed is nitrated by the nitrating
mixture and the isomeric nitro derivatives are then hydrolysed in the acidic medium as discussed under halogenation.
(v) Aniline does not undergo Friedel Crafts reaction. Actually, aniline being a Lewis base forms a complex with AICI3 which is a Lewis acid. The amino group is not in a position to activate the benzene ring towards electrophilic substitution i.e., alkylation or acylation which leads to Friedel Crafts reaction. Therefore, the reaction is not possible. The same problem arises in phenols as well.
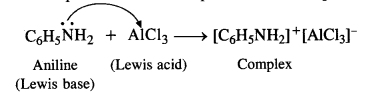
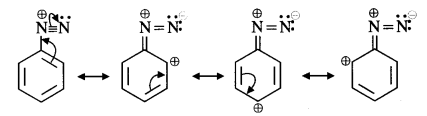
(vi) The diazonium salts of aromatic amines are more stable than those of aliphatic amines because these are resonance stabilised while no such resonance stabilisation is possible in the corresponding diazonium salts of aliphatic amines.
(vii) Gabriel phthalimide synthesis is generally preferred over other methods for the synthesis of primary aliphatic amines. Potassium phthalimide formed by reacting phthalimide with alcoholic KOH reacts with an alkyl halide such as C2H5-I to form N-alkyl derivative which undergoes hydrolysis to form the primary amine. However, no reaction is possible with aryl halide such as C6H5-I. Therefore, primary aromatic amines are not formed in the reaction.
Question 4.
Arrange the following:
(a) In decreasing order of the pKb values:
C2H5NH2, C6H5NHCH3, (C2H5)2NH and C6H5NH2
(b) In decreasing order of basic strength:
C6H5NH2, C6H5N(CH3)2, (C2H5)2NH and CH3NH2 (C.B.S.E. Delhi 2011, Haryana Board 2013, C.B.S.E. Outside Delhi 2015)
(c) Increasing order of basic strength
Aniline, p-nitroaniline and p-toluidine
(d) Decreasing order of basic strength in gas phase
C2H5NH2, (C2Hs)2NH, (C2H5)3N and NH3
(e) Increasing order of boiling point
C2H5OH, (CH3)2NH, C2HsNH2
(f) Increasing order of solubility in water
C6H5NH2, (C2Hs)2NH, C2H5NH2.
Answer:
From Kb and PKb values of some Amines:
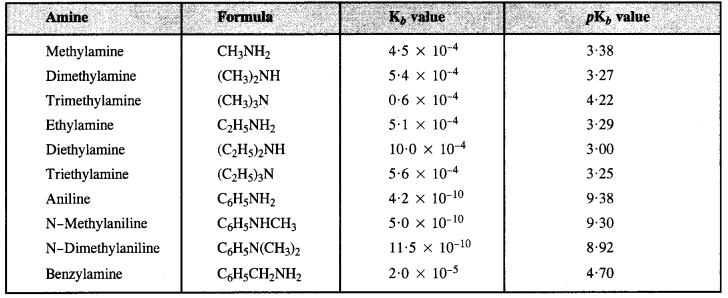
(a) The decreasing order of pKb values or increasing order of basic strength is:
C6H5NH2 > C6H5NHCH3 > C2H5NH2 > (C2H5)2NH
(b) The decreasing order of basic strength is:
(C2H5)2NH > CH3NH2 > C6H5N(CH3)2 > QH5NH2
(c) The increasing order of basic strength is:
p-nitroaniline < Aniline < p-Toluidine
(d) The decreasing order of basic strength in gaseous phase.
(C2H5)3N > (C2H5)2NH > C2H5NH2 > NH3
(e) Increasing order of boiling point is:
(C2H3)2NH < C2H5NH2 < C2H5OH
(f) Increasing order of solubility in water is :
C6H5NH2 < (C2H5)2NH < C2H5NH2.
Question 5.
How will you convert
(i) Ethanoic acid to methanamfrie,
(ii)Hexanenitrile to pentan-l-amine,
(iii)Methanol to ethanoic acid,
(iv) thatfaminft to methanamine,
(v)Ethanoic acid to propanoic acid,
(vi)Methanamine to ethanamine,
(vii)Nitromethane into dimethylamine,
(viii)Propanoic acid into ethanoic acid ?
Answer:
(i) Ethanoic acid to methanamfrie
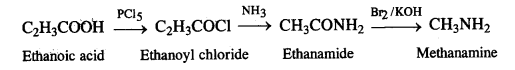
(ii) Hexanenitrile to pentan-l-amine
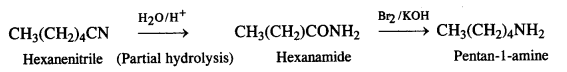
(iii) Methanol to ethanoic acid
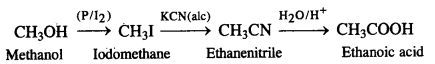
(iv) thatfaminft to methanamine
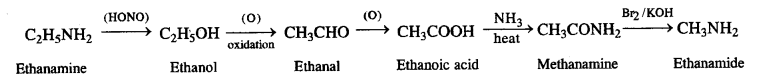
(v) Ethanoic acid to propanoic acid
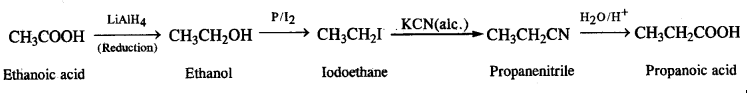
(vi) Methanamine to ethanamine
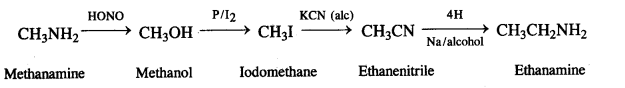
(vii) Nitromethane into dimethylamine
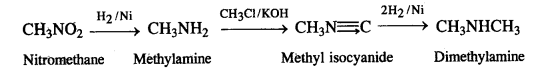
(viii) Propanoic acid into ethanoic acid
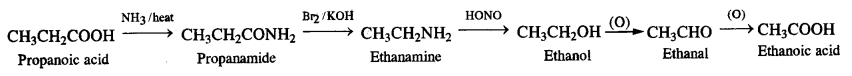
Question 6.
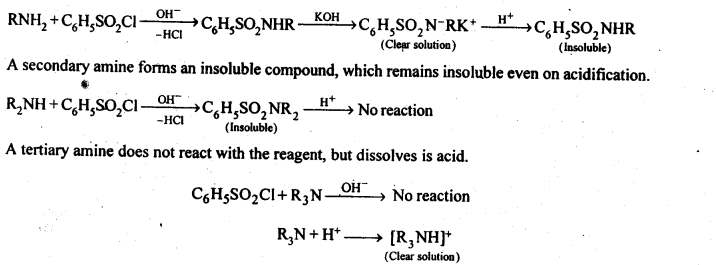
Describe a method for the identification of primary, secondary, and tertiary amines. Also, write chemical equations of the reactions involved.
Answer:
The three type of amines can be distinguished by Hinsberg test. In this test, the amine is shaken with benzene sulphonyl chloride (C6H5SO2Cl) in the presence of excess of aqueous NaOH or KOH. A primary amine reacts to give a clear solution, which on acidification yields an insoluble compound.
Question 7.
Write short notes on the following:
(i) Carbylamine reaction
(ii) Diazotisation
(iii) Hoffmann’s bromamide reaction
(iv) Coupling reaction
(v) Ammonolysis
(vi)Acetylation
(vii)Gabriel phthalimide synthesis. (C.B.S.E. Delhi 2011, C.B.S.E. Outside Delhi 2015)
Answer:
(i) Carbyl amine reaction:
Aliphatic and aromatic primary amines on heating with chloroform and ethanolic potassium hydroxide form isocyanides or carbylamines which are foul-smelling substances. Secondary and tertiary amines do not show this reaction. This reaction is known as carbylamine reaction or isocyanide test and is used as a test for primary amines.
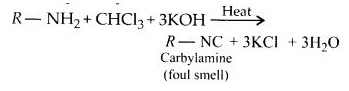
(ii) Diazotisation:
Primary aromatic amines such as aniline react with nitrous acid under ice-cold conditions (273 – 278 K) to form benzene diazonium salt. The reaction is known as diazotisation reaction.
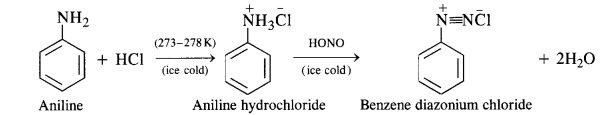
In case, the temperature is allowed to rise above 278 K, benzene diazonium chloride is decomposed by water to form phenol.
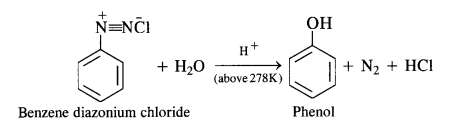
Aliphatic primary amines also react with nitrous acid to form alkyl diazonium salts in a similar manner. But these are quite unstable and decompose to form a mixture of alcohols, alkenes, and alkyl halides along with the evolution of N2 gas.
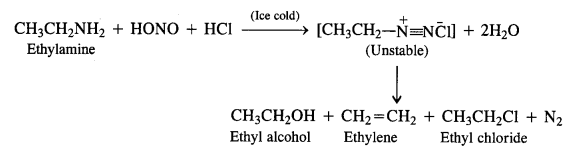
Aliphatic primary amines also react with nitrous acid to form alkyl diazonium salts in a similar manner. But these are quite unstable and decompose to form a mixture of alcohols, alkenes, and alkyl halides along with the evolution of N2 gas.
(iii) Hoffmann’s bromamide reaction:
By Hoffmann degradation of Acid Amides. (Hoffmann Bromamide Reaction). When a primary acid amide is heated with an aqueous or ethanolic solution of sodium hydroxide and bromine, it gives a primary amine with one carbon
atom less.
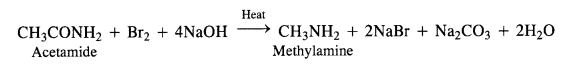
The reaction is, therefore, regarded as a degradation reaction. For example.
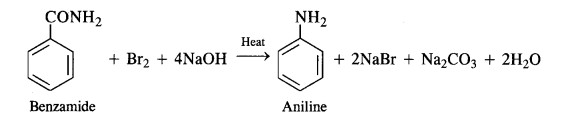
(iv) Coupling reaction:
The reaction of diazonium salts with phenols and aromatic amines to form azo compounds having an extended conjugated system with both aromatic rings joined through the — N = N — bond, is called coupling reaction. In this reaction; the nitrogen atoms of the diazo group are retained in the product. The coupling with phenols takes place in a mildly alkaline medium while that with amines occurs under faintly acidic conditions. For example;
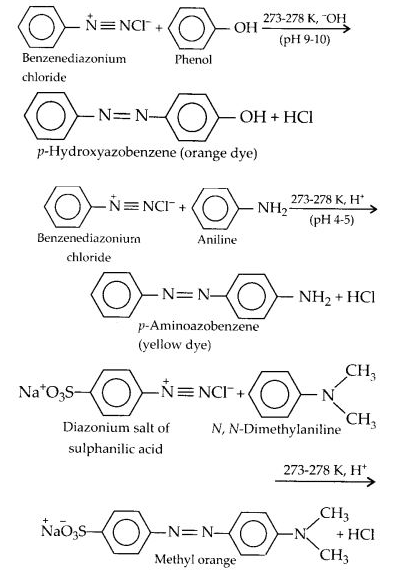
Coupling generally occurs at the p-position with respect to the hydroxyl or the amino group, if free, otherwise it takes place at the o-position.
(v) Ammonolysis:
The mechanism involves the nucleophilic attack of NH3 molecule (through lone pair) on alkyl halide by an SN2 mechanism. Amine salt is formed which reacts with ammonia to give primary amine and ammonium halide as follows:
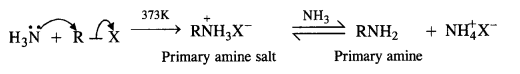
The primary amine formed now acts as the nucleophile and reacts with another molecule of the alkyl halide to form secondary amine.

The reaction is repeated to form tertiary amine and quaternary ammonium salt as follows :
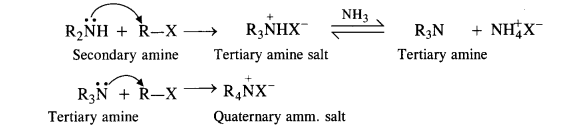
(vi) Acetylation:
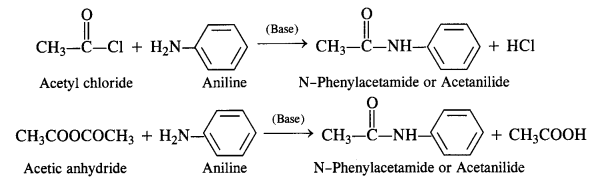
Acylation of Amines Both aliphatic and aromatic amines form acyl derivatives (substituted acid amides) with reagents such as acid chlorides, esters, or acid anhydrides. The acylation is carried out in the presence of a base stronger than pyridine (e.g., NaOH) which can remove the acid formed in the reaction by neutralising it.
(a) Acylation of Aliphatic Amines: Both primary and secondary aliphatic amines from acyl derivatives as follows:
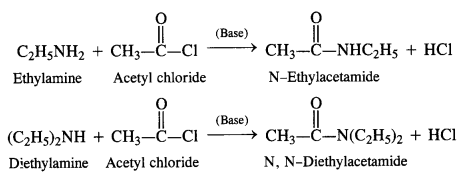
(b) Acylation of Aromatic Amines: Aromatic amines such as aniline can be acylated in the same manner with both acid
chloride and acid anhydride.
(vii) Gabriel’s phthalimide synthesis:
In this reaction phthalimide is converted into its potassium salt by treating it with alcoholic potassium hydroxide. Then potassium phthalimide is heated with an alkyl halide to yield an N-alkylpthalimide which is hydrolysed to phthalic acid and primary amine by alkaline hydrolysis
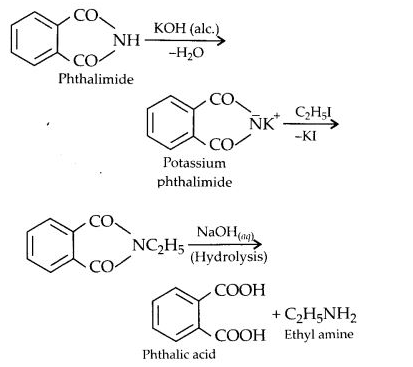
This synthesis is very useful for the preparation of pure aralkyl and aliphatic primary amines. However, aromatic primary amines cannot be prepared by this method.
Question 8.
Accomplish the following conversions:
(i) Nitrobenzene to benzoic acid
(ii) Benzene to m-bromophenol
(iii) Benzoic acid to aniline
(iv) Aniline to 2, 4, 6-tribromofluorobenzene
(v) Benzyl chloride to 2-phenylethanamine
(vi) Chlorobenzene to p-chloroaniline
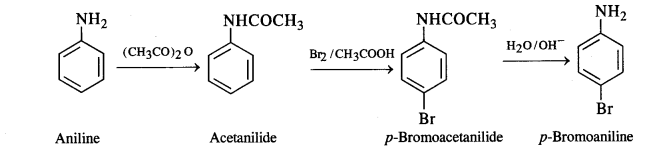
(vii) Aniline to p-bromoaniline
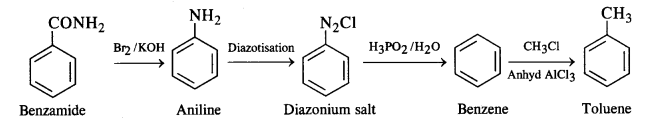
(viii)Benzamide to toluene
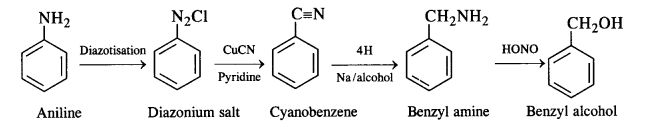
(ix)Aniline to benzyl alcohol.
Answer:
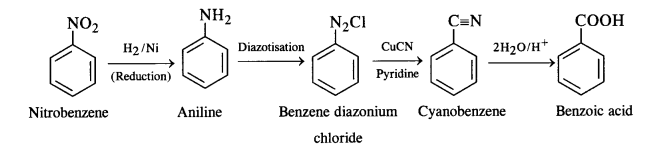
(i) Nitrobenzene to benzoic acid:
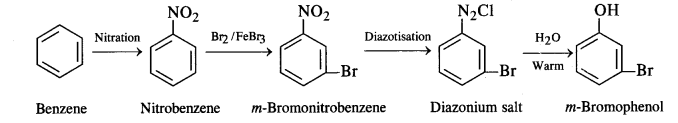
(ii) Benzene to m-bromophenol:
(iii) Benzoic acid to aniline:
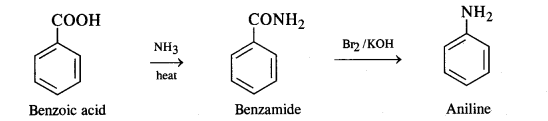
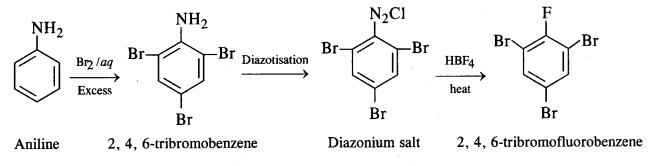
(iv) Aniline to 2, 4, 6-tribromofluorobenzene:
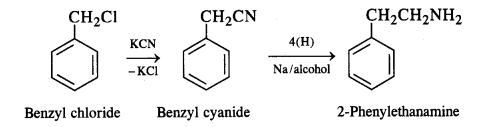
(v) Benzyl chloride to 2-phenylethanamine:
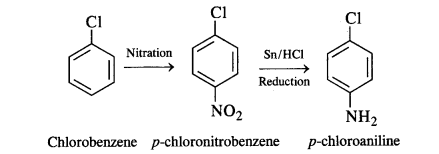
(vi) Chlorobenzene to p-chloroaniline:
(vii) Aniline to p-bromoaniline:
(viii)Benzamide to toluene :
(ix)Aniline to benzyl alcohol:
Question 9.
Give the structures of A, B, and C in the following reactions:
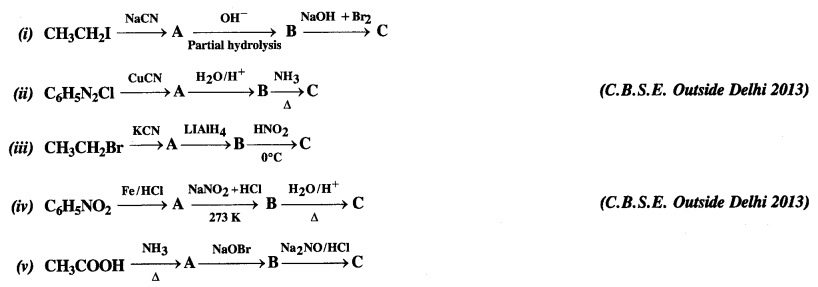
Answer:
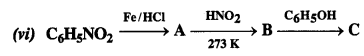
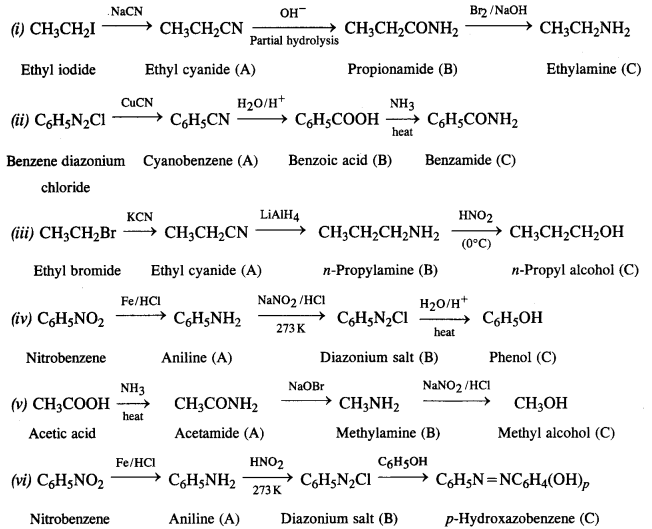
Question 10.
An aromatic compound ‘A’ on treatment with aqueous ammonia and heating forms compound ‘B’ which on heating with Br2 and KOH forms a compound ‘C’ of molecular formula C6H7N. Write the structures and IUPAC names of compounds A, B, and C.
Answer:
Since the compound ‘C’ with molecular formula C6H7N is formed from compound ‘B’ on treatment with Br2 KOH, therefore, compound ‘B’ must be an amide and ‘C’ must be an amine.
The only amine having the molecular formula C6H7N, i. e., C6H5NH2 is aniline.
Since ‘C’ is aniline, therefore, the die amide from which it is formed must be benzamide (C6H5CONH2). Thus, compound ‘B’ is benzamide. Since compound ‘B’ is formed from compound ‘A’ with aqueous ammonia and heating, therefore, compound ‘A’ must be benzoic acid.
Question 11.
Complete the following reactions:
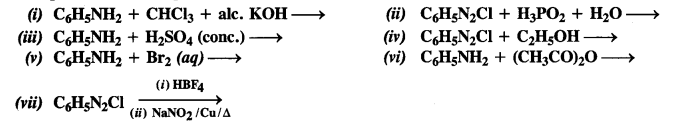
Answer:
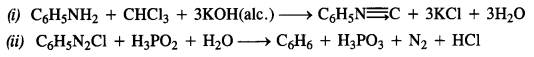
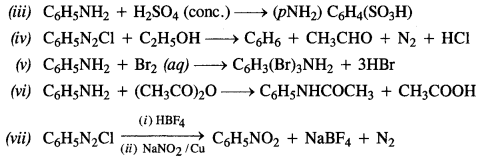
Question 12.
Why cannot aromatic amines be prepared by Gabriel’s phthalimide reaction? (C.B.S.E. Sample Question Paper 2012, H.P. Board2017)
Answer:
In Gabriel phthalimide reaction, the potassium salt of phthalimide is formed. It readily reacts with an alkyl halide to form the corresponding alkyl derivative.
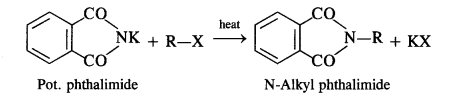
But it is not in a position to react with the aryl halide in case primary aromatic amine is to be prepared. Actually, the cleavage of C – X bond in haloarene or aryl halide is quite difficult due to partial double bond character. Therefore, aromatic amines cannot be prepared by this method.
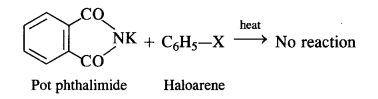
Question 13.
How do aromatic and aliphatic primary amines react with nitrous acid?
Answer:
Reaction with nitrous acid. All three types of amines, aliphatic as well as aromatic, react with nitrous acid under different conditions to form a variety of products. Since nitrous acid is highly unstable, it is prepared in situ by the action of dilute hydrochloric acid on sodium nitrite.
(a) Primary aliphatic amines react with nitrous acid at low temperature (cold conditions) to form primary alcohol and nitrogen gas accompanied by brisk effervescence. Nitrous acid is unstable in nature and is prepared in situ by reacting sodium nitrite with dilute hydrochloric acid. For example,
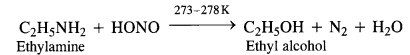
The reaction ¡s used as a rest for primary aliphatic amines as no other amine evolves nitrogen with nitrous acid.
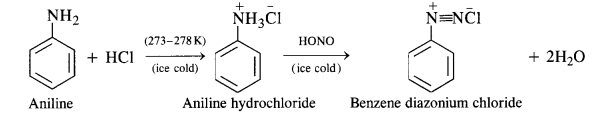
(b) Primary aromatic amines such as aniline react with nitrous acid under ice cold conditions (273—278 K) to form benzene diazonium salt. The reaction is known as diazotisation reaction.
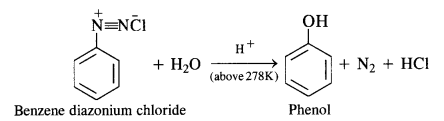
In case, the temperature is allowed to rise above 278 K, benzene diazonium chloride is decomposed by water to form phenol.
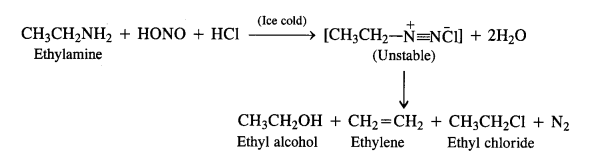
Aliphatic primary amines also react with nitrous acid to form alkyl diazonium salts in a similar manner. But these are quite unstable and decompose to form a mixture of alcohols, alkenes, and alkyl halides along with the evolution of N2 gas.
Question 14.
Give plausible explanation for each of the following :
(i) Why are amines less acidic than alcohols of comparable molecular masses ?
(ii) Why are primary amines higher boiling than tertiary amines ?
(iii) Why are aliphatic amines stronger bases than aromatic amines ? (H.P. Board 2008)
Answer:
(i) The acidic character in the both in cases is due to the release of H+ ion. Now, the anion in case of amine
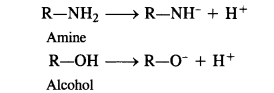
has a negative charge on the nitrogen atom while the anion formed in case of alcohol has negative charge on the oxygen atom. Since oxygen is more electronegative than nitrogen atom, the negative charge can be accomodated easily on oxygen than on nitrogen in these anions. In other words, RO” ion is more stable than RNH“ ion. Consequently, alcohol is a stronger acid than amine. Please remember that even alcohols are very weakly acidic so much so that they donot turn blue litmus red.
(ii) Primary amines are higher boiling than tertiary amines due to the presence of intermolecular hydrogen bonding in their molecules. Since tertiary amines (R3N) have no hydrogen atom present, these are not involved in any such hydrogen bonding. For example, the boiling point of n-butylamine (CH3CH2CH2CH2NH2) is 322 K while that of trimethylamine (CH3)3 N is 276 K.
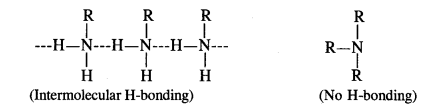
(iii) In the aromatic amines, (lie secondary and tertiary amines are more basic than aniline.
Actually, the basic strength or the electron releasing tendency of an amine depends upon the following factors.
- The ability of the nitrogen atom to donate a pair of electrons.
- The stability of cation by accepting the pair of electrons.
Any factor which tends lo increase the electron releasing tendency of amine or increase the stability of the cation, will tend to increase the basic strength of amine.
We hope the NCERT Solutions for Class 12 Chemistry Chapter 13 Amines help you. If you have any query regarding NCERT Solutions for Class 12 Chemistry Chapter 13 Amines, drop a comment below and we will get back to you at the earliest.