Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Nomenclature of Aldehydes and Ketones
We have already learnt the IUPAC system of nomenclature of organic compounds in XIth standard. Let us apply the rules to name the following compounds.
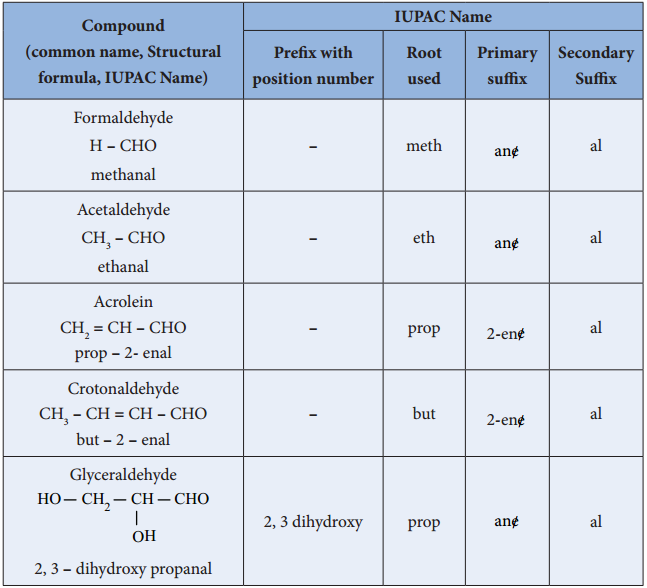
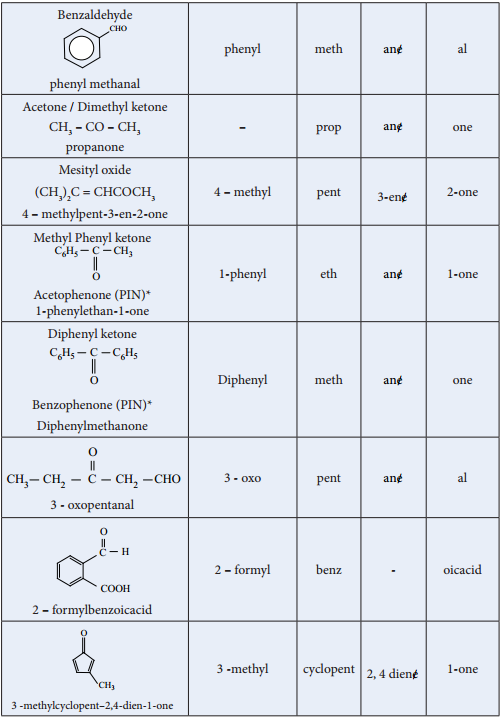
*PIN – Preferred IUPAC name
Aldehydes contain the carbonyl group bonded to at least one hydrogen atom. Ketones contain the carbonyl group bonded to two carbon atoms.
Naming Ketones
- Ketones take their name from their parent alkane chains.
- The common name for ketones are simply the substituent groups listed alphabetically +ketone.
They are named by finding the carbonyl group and identifying it with a location number, if necessary, then adding the suffix “- one.” The common name for ketones is determined by naming the alkyl groups attached to the carbonyl (in alphabetical order), then adding ‘ketone’.
For an aldehyde, drop the -e from the alkane name and add the ending -al. Methanal is the IUPAC name for formaldehyde, and ethanal is the name for acetaldehyde.
Nomeclature of ketone
The parent chain is numbered from the end that gives the carbonyl carbon the smaller number. The suffix -e of the parent alkane is changed to -one to show that the compound is a ketone.
Characteristics of Aldehydes and Ketones
Aldehydes and ketones are the class of organic compounds that have a carbonyl group i.e. carbon-oxygen double bond (-C=O). As they do not have any other reactive groups like -OH or -Cl attached to the carbon atom in the carbonyl group they are very simple compounds.
Functional Group of Ketone
Nomenclature of Aldehydes and Ketones. Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, C=O. The carbon atom of this group has two remaining bonds that may be occupied by hydrogen or alkyl or aryl substituents.
You will remember that the difference between an aldehyde and a ketone is the presence of a hydrogen atom attached to the carbon-oxygen double bond in the aldehyde. Ketones don’t have that hydrogen. Aldehydes are easily oxidized by all sorts of different oxidizing agents: ketones are not.
Aldehyde Formula
Aldehyde is a chemical compound with a functional group -CHO. The general formula of alkene is CnH2n+1 so the general formula for aldehyde will be CnH2n+1CHO or CnH2nO.
An aldehyde is similar to a ketone, except that instead of two side groups connected to the carbonyl carbon, they have at least one hydrogen (RCOH). The simplest aldehyde is formaldehyde (HCOH), as it has two hydrogens connected to the carbonyl group.
Combined with other functional group aldehydes and ketone are widespread in nature. Compounds such as cinnamaldehyde (cinnamon bark), vanillin (vanilla bean), Citra (lemongrass), helminthosporal (a fungal toxin), carvone (spearmint and caraway), camphor (camphor trees) are found chiefly in microorganisms or plants.