Occurrence And Extraction of Metals:
A major source of metals is the earth’s crust. Soluble salts of certain metals such as sodium chloride, magnesium chloride, etc., are also found in seawater. Minerals: The inorganic elements or compounds which occur naturally in the earth’s crust are known as minerals, for example, zinc blende, cinnabar, etc. Ores: Those minerals from which a metal can be profitably extracted are called ores.
Gangue: The impurities like sand and rocky materials which are present in the ore are called gangue.
Occurrence of Metals:
(A) The metals at the bottom of the activity series (Au, Ag, Pt etc) are the least reactive and are often found in a free state.
(B) The metals in the middle of the activity series (Zn, Fe, Pb, etc) are moderately reactive and are found in the earth’s crust mainly as oxides, sulphides or carbonates.
(C) Metals at the top of the activity series (K, Na, Ca, Mg, Al) are very reactive and are never found as free elements.
Important
The ores of many metals are oxides because oxygen is a very reactive element and is very abundant on the earth.
Extraction of Metals:
The extraction of metals and the refining of them for use is known as metallurgy. The process of metallurgical operations consists of mainly three steps:
- Enrichment of ores
- Reduction
- Refining.
![]()
Steps involved in the extraction of pure metals from ores
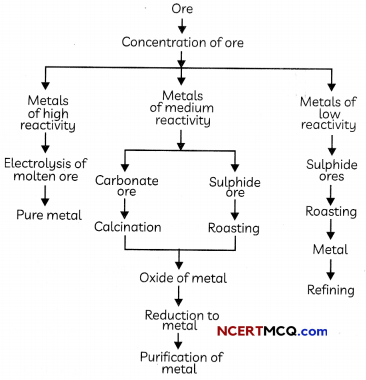
Enrichment of Ores
The removal of impurities like sand, soil etc from the ore prior to the extraction of the metals is called enrichment of ores. The processes used for removing the gangue from the ore are based on the differences between the physical or chemical properties of the ore and the gangue.
Reduction of Ore to Metal:
Reduction: The process of obtaining metals from their oxides is known as reduction. Metal sulphides and carbonates are first converted into metal oxides as it is easier to reduce metal oxides as compared to metal sulphides and carbonates.
- Extracting Metals Low in the Activity Series: Metals which are low in the activity series are very unreactive. Their oxides can be reduced to metals by heating alone.
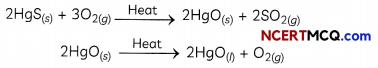
Examples: - Extraction of Mercury: Cinnabar (HgS) is an ore of Mercury, which is first converted to the mercuric oxide by heating and then it is reduced to mercury on further heating:
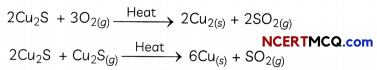
- Extraction of Copper: Copper which is found as Cu2S is obtained from its ore by heating in air:
2. Extracting Metals in the Middle of the Activity Series
Metals in the middle of the activity series such as iron, zinc, lead, copper etc are moderately reactive and are usually present as sulphides or carbonates in nature.
- Roasting: The process of converting sulphide ores into oxides by strongly heating in presence of excess of air is known as roasting.
2ZnS(s) + 3O2(g) → 2ZnO(S) + 2SO2(g) - Calcination: The process of converting carbonate ores into oxides by strongly heating in absence of excess of air is known as calcination.
ZnCO3(s) → ZnO(s) + CO2(g)
![]()
The metal oxides are then reduced to the corresponding metals by using suitable reducing agents such as carbon or by using displacement reactions with a more reactive metal.
Methods of Reduction:
| Metal Type | Method of Reduction |
| Unreactive metals which are Low in the activity series, like mercury | Oxides of these metals are reduced by heating alone. Cinnabar (HgS) is heated in air and converted to mercuric oxide (HgO): 2HgS(S) + 3O2(g) → 2HgO(s) + 2SO2(g)
Mercuric oxide is then reduced to mercury on further heating: 2HgO(s) → 2Hg(l) + O2(g) |
| Moderately reactive metals which are in the middle of the activity series, such as iron, zinc, lead, copper etc | Oxides of these metals are reduced by heating with carbon. ZnO(s) + C(s) → Zn(s) + CO(g) |
| Very reactive
metals which are high up in the activity series, such as sodium, magnesium, calcium etc |
Sometimes, displacement reactions can also be used. The highly reactive metals such as sodium, calcium, aluminium, etc, are used as reducing agents.
3Mn02(S) + 4Al(s) → 3Mn(l) + 2Al2O3(S) + Heat |
| The oxides of these metals are reduced by electrolytic reduction, i.e., by the electrolysis of their fused chlorides. The metals are deposited at the cathode and chlorine is liberated at the anode.
At cathode: Na+ → e– + Na |
Thermite Reactions: The reactions of metal oxides with aluminium are highly exothermic and the amount of heat evolved is so large that the metals are produced in the molten state. The reaction of iron oxide (Fe203) with aluminium is used to join railway tracks or cracked machine parts.
Fe2O3(S) + 2Al(S) → 2Fe(l) + Al2O3(s)
3. Extracting Metals Towards the Top of the Activity Series
The highly reactive metals are extracted by the electrolysis of their molten chlorides or oxides. These oxides are very stable and cannot be reduced by the most common reducing agent carbon as these metals have more affnity for oxygen than carbon.
Extraction of Sodium Metal: Sodium metal is extracted by the electrolysis of molten sodium chloride. When electric current is passed through molten sodium chloride, it decomposes to form sodium metal and chlorine gas:
2NaCl → 2Na + Cl2
The reactions taking place at the two electrodes are given below:
At the Cathode: 2Na+ + 2e– → 2Na
At the Anode: 2Cl– + 2e– → Cl2
![]()
Example 1.
Which chemical process is used for obtaining a metal from its oxide?
Answer:
The chemical process used for obtaining a metal from its oxide is reduction. Metal oxides can be reduced to metal either by heating alone (less reactive metals) or by heating with carbon (moderately reactive metals).
Example: Reduction of HgO (oxide of less reactive metal) by heating alone:
2HgO(s) → 2Hg(l) + O2(g)
Reduction of ZnO (oxide of moderately reactive metal) by heating with carbon:
ZnO(s) + C(s) → Zn(s) + CO(g)
Refining of Metals
The process of purification of impure metals is known as refining of metals and the method used for refining an impure metal depends upon:
- Nature of the element
- Nature of impurities present in it.
Electrolytic refining of copper: The metaLs such as copper, zinc, nickel, silver, gold, etc., are refined electrolytically.
The impure metal is made as anode and a thin strip of pure metal is made as cathode A solution of the metal salt is used as an electrolyte.
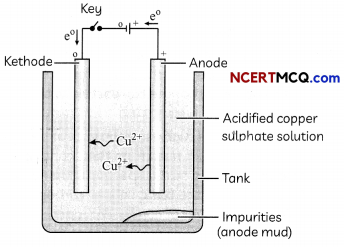
Electrolytic refining of copper
On passing a current through the electrolyte, the pure metal from the anode dissolves into the electrolyte. An equivalent amount of pure metal from the electrolyte is deposited on the cathode.
The solid impurities go into the solution and the insoluble impurities settle down at the bottom of the anode and are known as anode mud.
At anode: Cu → Cu2+ + 2e–
At cathode: Cu2+ + 2e– → Cu
Anode: A thin block of impure metal is connected to the positive terminal of the battery.
Cathode: A thin strip of pure metal is connected to the negative terminal of the battery.
Electrolyte: A water-soluble salt of the metal to be refined is taken as the electrolyte.