Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Periodic Trends in Chemical Properties:
So far, we have studied the periodicity of the physical properties such as atomic radius, ionisation enthalpy, electron gain enthalpy and electronegativity. In addition, the chemical properties such as reactivity, valence, oxidation state etc… also show periodicity to certain extent.
In this section, we will discuss briefly about the periodicity in valence (oxidation state) and anomalous behaviour of second period elements (diagonal relationship).
Valence or Oxidation States
The valence of an atom is the combining capacity relative to hydrogen atom. It is usually equal to the total number of electrons in the valence shell or equal to eight minus the number of valence electrons. It is more convenient to use oxidation state in the place of valence.
Periodicity of Valence or Oxidation States
The valence of an atom primarily depends on the number of electrons in the valence shell. As the number of valence electrons remains same for the elements in same group, the maximum valence also remains the same. However, in a period the number of valence electrons increases, hence the valence also increases.
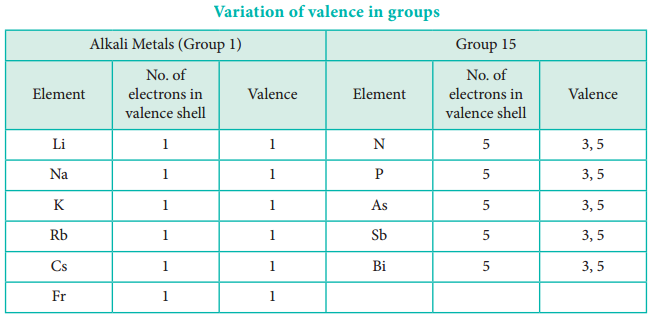
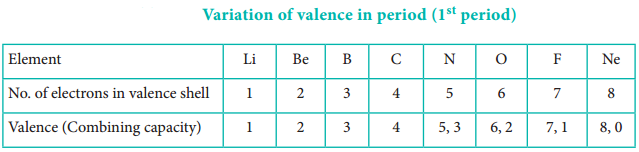
In addition to that some elements have variable valence. For example, most of the elements of group 15 which have 5 valence electrons show two valences 3 and 5. Similarly transition metals and inner transition metals also show variable oxidation states.
Anomalous Properties of Second Period Elements:
As we know, the elements of the same group show similar physical and chemical properties. However, the first element of each group differs from other members of the group in certain properties. For example, lithium and beryllium form more covalent compounds, unlike the alkali and alkali earth metals which predominantly form ionic compounds.
The elements of the second period have only four orbitals (2s & 2p) in the valence shell and have a maximum co-valence of 4, whereas the other members of the subsequent periods have more orbitals in their valence shell and shows higher valences. For example, boron forms BF4– and aluminium forms AlF63-.
Diagonal Relationship
On moving diagonally across the periodic table, the second and third period elements show certain similarities. Even though the similarity is not same as we see in a group, it is quite pronounced in the following pair of elements.
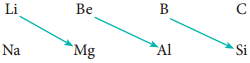
The similarity in properties existing between the diagonally placed elements is called ‘diagonal relationship’.
Periodic Trends and Chemical Reactivity:
The physical and chemical properties of elements depend on the valence shell electronic configuration as discussed earlier. The elements on the left side of the periodic table have less ionisation energy and readily lose their valence electrons.
On the other hand, the elements on right side of the periodic table have high electron affinity and readily accept electrons. As a consequence of this, elements of these extreme ends show high reactivity when compared to the elements present in the middle. The noble gases having completely filled electronic configuration neither accept nor lose their electron readily and hence they are chemically inert in nature.
The ionisation energy is directly related to the metallic character and the elements located in the lower left portion of the periodic table have less ionisation energy and therefore show metallic character. On the other hand the elements located in the top right portion have very high ionisation energy and are nonmetallic in nature.
Let us analyse the nature of the compounds formed by elements from both sides of the periodic table. Consider the reaction of alkali metals and halogens with oxygen to give the corresponding oxides.
4 Na + O2 → 2 Na2O
2 Cl2 + 7O2 → 2 Cl2O7
Since sodium oxide reacts with water to give strong base sodium hydroxide, it is a basic oxide. Conversely Cl2O7 gives strong acid called perchloric acid upon reaction with water So, it is an acidic oxide.
Na2O + H2O → 2NaOH
Cl2O7 → 2 Cl2O7
Thus, the elements from the two extreme ends of the periodic table behave differently as expected. As we move down the group, the ionisation energy decreases and the electropositive character of elements increases. Hence, the hydroxides of these elements become more basic. For example, let us consider the nature of the second group hydroxides:
Be(OH)2 amphoteric; Mg(OH)2 weakly basic; Ba(OH)2 strongly basic
Beryllium hydroxide reacts with both acid and base as it is amphoteric in nature.
Be(OH)2 + 2HCl → BeCl2 + 2H2O
Be(OH)2+ 2 NaOH → Na2BeO2 + 2H2O.