Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Physical Properties of Carboxylic Acids
1. Aliphatic carboxylic acid upto nine carbon atoms are colour less liquids with pungent odour. The higher members are odourless wax like solids.
2. Carboxylic acids have higher boiling point than aldehydes, ketones and even alcohols of comparable molecular masses. This is due to more association of carboxylic acid molecules through intermolecular hydrogen bonding.
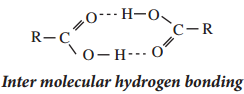
In fact, most of the carboxylic acids exist as dimer in its vapour phase.
3. Lower aliphatic carboxylic acids (up to four carbon) are miscible with water due to the formation of hydrogen bonds with water. Higher carboxylic acid are insoluble in water due to increased hydrophobic interaction of hydrocarbon part. The simplest aromatic carboxylic acid, benzoic acid is insoluble in water.
4. Vinegar is 6 to 8% solution of acetic acid in water. Pure acetic acid is called glacial acetic acid. Because it forms ice like crystal when cooled. When aqueous acetic acid is cooled at 289.5 K, acetic acid solidifies and forms ice like crystals, where as water remains in liquid state and removed by filtration. This process is repeated to obtain glacial acetic acid.
- Carboxylic acids have high boiling points compared to other substances of comparable molar mass. Boiling points increase with molar mass.
- Carboxylic acids having one to four carbon atoms are completely miscible with water. Solubility decreases with molar mass.
Carboxylic acids are soluble in water. Carboxylic acids do not dimerise in water, but forms hydrogen bonds with water. Carboxylic acids are polar and due to the presence of the hydroxyl in the carboxyl group, they are able to form hydrogen bonds with water molecules.
The solubility of compounds containing the carboxyl functional group in water depends on the size of the compound. The smaller the compound (the shorter the R group), the higher the solubility. The boiling point of a carboxylic acid is generally higher than that of water.
Larger carboxylic acids are solids with low melting points. There are a great many aromatic carboxylic acids, which are all crystalline solids. Carboxylic acids can form intermolecular hydrogen bonds and thus have relatively high melting and boiling points compared to other organic compounds that cannot hydrogen bond.
- Carboxyl group comprises electronegative oxygen double bond to a carbon atom.
- A compound comprising a carboxyl group will possess a high melting point, hydrophilic centres, and boiling point.