Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Position in Periodic Table
The hydrogen has the electronic configuration of 1s1 which resembles with ns1 general valence shell configuration of alkali metals and shows similarity with them as follows:
- It forms unipositive ion (H+) like alkali metals (Na+, K+, Cs+)
- It forms halides (HX), oxides (H2O), peroxides (H2O2) and sulphides (H2S) like alkali metals (NaX, Na2O, Na2O2, Na2S)
- It also acts as a reducing agent.
However, unlike alkali metals which have ionization energy ranging from 377 to 520 kJ mol-1, the hydrogen has 1, 314 KJ mol-1 which is much higher than alkali metals. Like the formation of halides (X–) from halogens, hydrogen also has a tendency to gain one electron to form hydride ion (H–) whose electronic configuration is similar to the noble gas, helium. However, the electron affinity of hydrogen is much less than that of halogen atoms. Hence, the tendency of hydrogen to form hydride ion is low compared to that of halogens to form the halide ions as evident from the following reactions:
½ H2 + e– → H– ΔH = + 36 kcal mol-1
½ Br2 + e– → Br– ΔH = – 55 kcal mol-1
Since, hydrogen has similarities with alkali metals as well as the halogens; it is difficult to f nd the right position in the periodic table. However, in most of its compounds hydrogen exists in +1 oxidation state. Therefore, it is reasonable to place the hydrogen in group 1 along with alkali metals as shown in the latest periodic table published by IUPAC.
Isotopes of Hydrogen
Hydrogen has three naturally occurring isotopes, viz., protium (1H1 or H), deuterium (1H2 or D) and tritium (1H3 or T). Protium (1H1) is the predominant form (99.985 %) and it is the only isotope that does not contain a neutron.
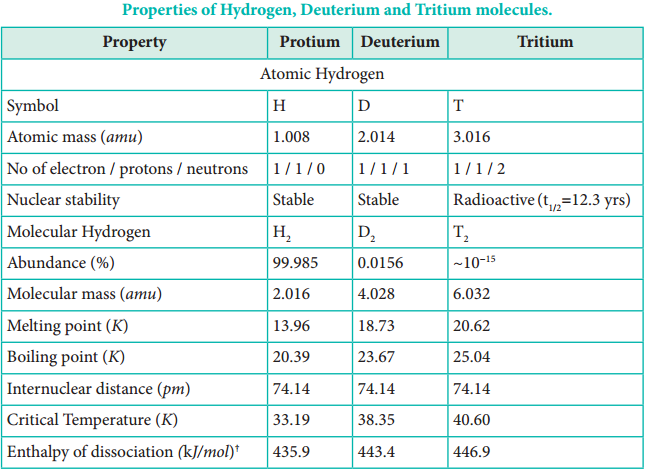
Deuterium, also known as heavy hydrogen, constitutes about 0.015 %. The third isotope, tritium is a radioactive isotope of hydrogen which occurs only in traces (~1 atom per 1018 hydrogen atoms). Due to the existence of these isotopes naturally occurring hydrogen exists as H2, HD, D2, HT, T2 and DT. The properties of these isotopes are shown in Table 4.1.
Ortho and Para-Hydrogen:
In the hydrogen atom, the nucleus has a spin. When molecular hydrogen is formed, the spins of two hydrogen nuclei can be in the same direction or in the opposite direction as shown in the figure. These two forms of hydrogen molecules are called ortho and para hydrogens respectively.

At room temperature, normal hydrogen consists of about 75% ortho-form and 25% paraform. As the ortho-form is more stable than para-form, the conversion of one isomer into the other is a slow process. However, the equilibrium shift in favour of para hydrogen when the temperature is lowered.
The para-form can be catalytically transformed into ortho-form using platinum or iron. Alternatively, it can also be converted by passing an electric discharge, heating above 800°C and mixing with paramagnetic molecules such as O2, NO, NO2 or with nascent/atomic hydrogen.
Ortho and para hydrogen are similar in chemical properties but differ in some of the physical properties. For example, the melting point of para hydrogen is 13.83 K while that of ortho hydrogen 13.95 K; boiling point of para hydrogen is 20.26 K while that of ortho hydrogen 20.39 K. Since the nuclear spins are in opposite directions the magnetic moment of para hydrogen is zero and ortho hydrogen has magnetic moment twice that of a proton.