Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Position of D – Block Elements in the Periodic Table
We have already learnt the periodic classification of elements in XI std. the transition metals occupy from group – 3 to group – 12 of the modern periodic table.
Figure 4.1 – Position of d – block elements in the periodic table
D – Block elements composed of 3d series (4th period) Scandium to Zinc (10 elements), 4d series (5th period) Yttrium to Cadmium (10 elements) and 5d series (6th period) Lanthanum, Haffinium to mercury. As we know that the group-12 elements Zinc, Cadmium and Mercury do not have partially filled d-orbital either in their elemental state or in their normal oxidation states.
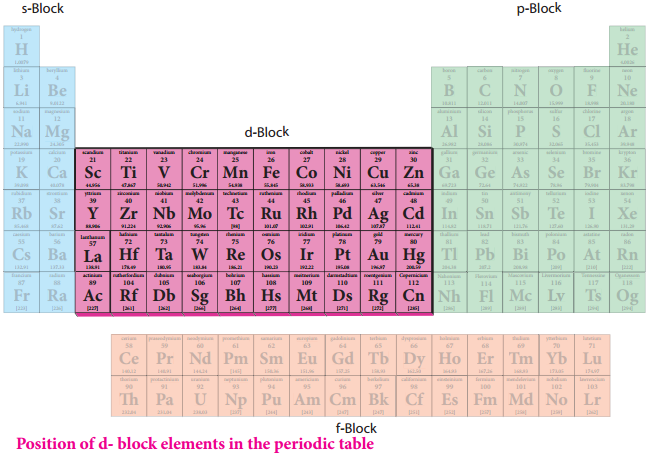
However they are treated as transition elements, because their properties are an extension of the properties of the respective transition elements. As per the IUPAC definition, the seventh period elements, starting from Ac, Rf to Cn also belong to transition metals. All of them are radioactive. Except Actinium; all the remaining elements are synthetically prepared and have very low half life periods.
The d-block elements are found in the middle of the period table. The d-block elements are called transition metals and have valence electrons in d orbital’s. The f-block elements,found in the two rows at the bottom of the periodic table, are called inner transition metals and have valence electrons in the f-orbital’s.
Transition elements are the elements that are found in Groups 3-12 (old groups IIA-IIB) on the periodic table (salmon-colored block in the middle of the table).
The Periodic Table provides a section for each of these groups of orbitals. The 10 electrons of the five d orbitals are filled by the elements found in the dropped central section of the table. This section is referred to as the d block elements, or the transition metals.
The d-block of the periodic table contains the elements of the groups 3-12 in which the d orbitals are progressively filled in each of the four long periods. The f-block consists of elements in which 4 f and 5 f orbitals are progressively filled. They are placed in a separate panel at the bottom of the periodic table.
The d-block elements are called transition elements because they exhibit transitional behaviour between s block and p-block elements. Their properties are transitional between highly reactive metallic elements of s block which are ionic in nature and the elements of p-block which are covalent in nature.
According to Aufbau principle , electrons first occupy the lowest energy orbital available to them and enter into higher energy orbitals only after the lower energy orbitals are filled. Therefore, 3d orbital is higher in energy than 4s. And hence electrons fill up in 4s before filling up in 3d.
The p sublevel has 3 orbitals, so can contain 6 electrons max. The d sublevel has 5 orbitals, so can contain 10 electrons max. And the 4 sublevel has 7 orbitals, so can contain 14 electrons max. In the picture below, the orbitals are represented by the boxes.
D-Block Elements:
Elements in which the last electron enters any one of the five d-orbitals of their respective penultimate shells are called d-block elements. The Importance of d-block Transition Metals. Transition metals, for the most part, are good conductors. They are also malleable, ductile, lustrous, and sliver-white in color. The d-block metals, and some of it’s key alloys, shaped the Bronze Age, Iron Age, and most importantly the steel age.
The p-block elements are found on the right side of the periodic table. They include the boron, carbon, nitrogen, oxygen and flourine families in addition to the noble gases. The noble gases have full p-orbital’s and are nonreactive.
In chemistry and atomic physics, the main group is the group of elements (sometimes called the representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, and fluorine as arranged in the periodic table of the elements.
Chlorine is in group 17 of periodic table, also called the halogens, and is not found as the element in nature – only as a compound. The most common of these being salt, or sodium chloride, and the potassium compounds sylvite (or potassium chloride) and carnallite (potassium magnesium chloride hexahydrate).