Learninsta presents the core concepts of Biology with high-quality research papers and topical review articles.
Redox Reactions
NAD+ + 2e– + 2H+ → NADH + H+
FAD + 2e– + 2H+ → FADH2
When NAD+ (Nicotinamide Adenine Dinucleotide-oxidised form) and FAD (Flavin Adenine Dinucleotide) pick up electrons and one or two hydrogen ions (protons), they get reduced to NADH + H+ and FADH2 respectively.
When they drop electrons and hydrogen off they go back to their original form. The reaction in which NAD+ and FAD gain (reduction) or lose (oxidation) electrons are calledredox reaction (Oxidation reduction reaction). These reactions are important in cellular respiration.
An oxidation-reduction (redox) reaction is a type of chemical reaction that involves a transfer of electrons between two species. An oxidation-reduction reaction is any chemical reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron.
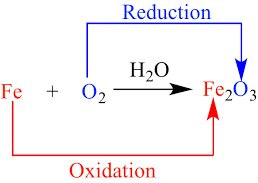
The reaction in which one substance gets oxidised and other gets reduced is known as redox reaction. Example: ZnO + C → Zn + CO. Here, C is oxidised to CO because oxygen is being added and ZnO is reduced to Zn because oxygen is being removed. Therefore, it is a redox reaction.
Keep this in mind as we look at the five main types of redox reactions: combination, decomposition, displacement, combustion, and disproportion. Combination. Combination reactions “combine” elements to form a chemical compound.
- Decomposition
- Displacement
- Combustion
- Disproportionation
Because any loss of electrons by one substance must be accompanied by a gain in electrons by something else, oxidation and reduction always occur together. As such, electron-transfer reactions are also called oxidation-reduction reactions, or simply redox reactions.
Remember that although redox reactions are common and plentiful, not all chemical reactions are redox reactions. All redox reactions involve complete or partial transfer of electrons from one atom to another. Both reactions above are examples of oxidation-reduction reactions.
Oxidation-reduction reaction, also called redox reaction, any chemical reaction in which the oxidation number of a participating chemical species changes.
Write the oxidation and reduction half-reactions for the species that is reduced or oxidized. Multiply the half-reactions by the appropriate number so that they have equal numbers of electrons. Add the two equations to cancel out the electrons. The equation should be balanced.
Double-replacement reactions such as the one below are not redox reactions because ions are simply recombined without any transfer of electrons. Note that the oxidation numbers for each element remain unchanged in the reaction.