Soaps
The molecules of soap are sodium or potassium salts of long-chain carboxylic acids.
A soap molecule has:
- Ionic (hydrophilic) part
- Long hydrocarbon chain (hydrophobic) part
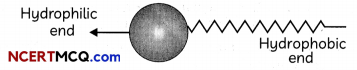
Structure of soap molecule
![]()
Example 1.
What change will you observe if you test soap with litmus paper (red and blue)?
Answer:
When soap is tested with blue and red litmus paper, it changes the colour of red litmus to blue but has no effect on blue litmus paper as soap is alkaline in nature.
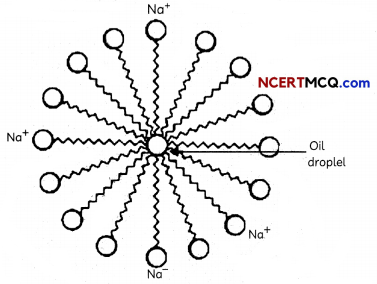
Micelles:
- Most dirt is oily in nature and as we know, oil does not dissolve in water.
- Soaps are molecules in which the two ends have differing properties, one is hydrophilic, that is, it dissolves in water, while the other end is hydrophobic, that is, it dissolves in hydrocarbons.
- When soap is at the surface of water, the hydrophobic ‘tail’ of soap will not be soluble in water and the soap will align along the surface of water with the ionic end in water and the hydrocarbon ‘tail’ protruding out of water.
- Inside water, these molecules have a unique orientation that keeps the hydrocarbon portion out of the water.
- The soap molecules, thus form structures called micelles (Figure) where one end of the molecules is towards the oil droplet while the ionic-end faces outside.
- This forms an emulsion in water. The soap micelle thus helps in dissolving the dirt in water and we can wash our clothes clean (Figure below).
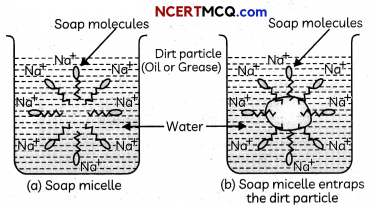
Cleansing Action of Soap
- Soap in theforn, of a micelle is able to clean, since the oily dirt wiLt be collected in the centre of the micelle.
- The micelles stay in soLution as a colloid and will not come together to precipitate because of on ion repulsion.
- Thus, the dirt suspended in the micelles is also easily rinsed away. The soap micelles ore large enough to scatter Light. Hence a soap solution appears cloudy.
Cleansing Action of Soap
Detergents
Detergents are sodium salts of sulphonic acids or ammonium salts with chlorides or bromide ions. Examples of synthetic detergents are: Sodium n-dodecyl benzene sulphonate and Sodium n-dodecyl sulphate.
![]()
Example 2.
Case Based:
Two students performed the activities to study the action of soap and detergents in soft and hard water. The first student took about 10 mL of distilled water (or rainwater) and 10 mL of hard water (from a tubewell or hand-pump) in separate test tubes and added a couple of drops of soap solution to both. He then shook the test tubes vigorously for an equal period of time. The second student took two test tubes with about 10 mL of hard water in each and added five drops of soap solution to one and five drops of detergent solution to the other and shook both test tubes for the same period.
(A) The observations recorded by the first student on adding a few drops of soap solution to 10 mL distilled water and hard water in separate test tubes is given below:
| Distilled Water | Hard Water |
| 1. Lot of foam observed | 1. Lot of foam observed |
| 2. Curdy precipitate observed | 2. Lot of foam observed |
| 3. Lot of foam observed | 3. Curdy precipitate observed |
| 4. Curdy precipitate observed | 4. Curdy precipitate observed |
Select the correct observation
Answer:
(c) Distilled water: Lot of foam observed; Hard water: Curdy precipitate observed Explanation: Soap forms lot of foam with distilled water but forms a curdy white precipitate known as scum when mixed with hard water. This is because the soap molecules react with the calcium and magnesium ions present in hard water to form the scum.
(B) The second student recorded the following observations after adding five drops of soap solution and detergent solution to two test tubes containing hard water.
(I) Both the test tube had an equal amount of foam
(II) The test tube in which soap was added had less amount of foam
(III) The test tube in which detergent was added had less amount of foam
(IV) A curdy solid was formed in the test tube in which soap was added
Select the incorrect observations. I and III
(a) Both (I) and (III)
(b) Both (I) and (IV)
(c) Both (II) and (IV)
(d) Both (III) and (IV)
Answer:
(a) Both (I) and (III)
Explanation: Soaps form an insoluble precipitate in hard water Whereas detergents are effective even in hard water as they do not form insoluble precipitates with the calcium and magnesium ions in hard water.
(C) What will be observed while using soap in water containing calcium sulfate?
Answer:
When we use soap in water containing calcium sulfate, soap forms foam with difficulty but forms an insoluble substance called scum as soap molecules react with the calcium salt found in hard water.
(D) How are soaps and detergents different structurally?
Answer:
The molecules of soap are sodium or potassium salts of long-chain carboxylic acids whereas detergents are generally ammonium or sulphonate salts of long-chain carboxylic acids.
(E) Assertion (A): Soaps are not effective in hard water
Reason (R): The charged ends of detergents do not form insoluble precipitates with the calcium and magnesium ions in hard water.
For the following questions, two statements are given one labeled Assertion (A) and the other labeled Reason (R). Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below:
(a) Both (A) and (R) are true and (R) is the correct explanation of the assertion.
(b) Both (A) and (R) are true, but (R) is not the correct explanation of the assertion.
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true.
Answer:
(b) Both (A) and (R) are true, but (R) is not the correct explanation of the (A).
Explanation: Soaps are not effective in hard water as soap molecules react with the calcium and magnesium salts present in hard water, such as CaSO4, MgSO4, CaCl2 and MgCl2, and forms an insoluble substance known as scum.
However, as the structure of detergents is different from that of soap, the charged ends of detergents do not form insoluble precipitates with the calcium and magnesium ions in hard water.
Effectiveness of Soaps and Detergents in Hard Water
Soap is not effective in hard water as soap reacts with the calcium and magnesium salts, which cause the hardness of water, and forms foam with difficulty and an insoluble substance (scum) remains after washing with water.
Detergents are effective in hard water as the charged ends of these compounds do not form insoluble precipitates with the calcium and magnesium ions in hard water. Thus, they remain effective in hard water. Detergents are usually used to make shampoos and products for cleaning clothes.
![]()
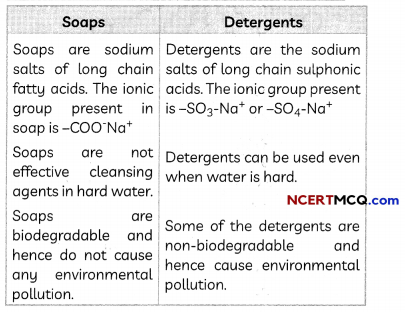
Differences between Soaps and Detergents