Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Structure of Carboxyl Group:
Th carboxyl group represent a planar arrangement of atoms. In – COOH group, the centre carbon atom and both the oxygen atoms are in sp hybridisation. The three sp2 hybrid orbitals of the carbon atom overlap.
The two sp2 – hybridised orbitals of the carboxyl carbon overlap with one sp2 hybridised orbital of each oxygen atom while the third sp2 hybridised orbital of carbon overlaps with either a s – orbital of H – atom or a sp2 – hybridised orbital of C – atom of the alkyl group to form three s – bonds. Each of the two oxygen atoms and the carbon atom are left with one unhybridised p – orbital which is perpendicular to the s – bonding skeleton.
All these three p – orbitals being parallel overlap to form a π – bond which is partly delocalized between carbon and oxygen atom on one side, and carbon and oxygen of the OH group on the other side. In other words, RCOOH may be represented as a resonance hybrid of the following two canonical structures.
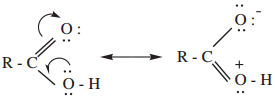
The carboxylic carbon is less electrophilic than carbonyl carbon because of the possible resonance structure. i.e., delocalisation of lone pair electrons from the oxygen in hydroxyl group.
Carboxyl group is a functional organic compound. In this structure of a carboxyl group, a carbon atom is attached to an oxygen atom with the help of a double bond. The carboxyl group ionizes and releases the H atom present in the hydroxyl group part as a free H+ ion or a proton.
Carboxylic acid, any of a class of organic compounds in which a carbon (C) atom is bonded to an oxygen (O) atom by a double bond and to a hydroxyl group (- OH) by a single bond. A fourth bond links the carbon atom to a hydrogen (H) atom or to some other univalent combining group.
The Carboxyl group contains a double bond of electronegative oxygen to a carbon atom. As a result, the polarity of a bond will increase. A compound containing a carboxyl group should possess hydrophilic centres with a high melting point and boiling point.
Carboxyl groups are functional groups with a carbon atom double-bonded to an oxygen atom and single bonded to a hydroxyl group. Ionized carboxyl groups act as acids, require less energy and are more stable. Electron sharing between oxygen atoms on ionized carboxyl groups increases stability.
A carboxyl group (COOH) is a functional group consisting of a carbonyl group (C=O) with a hydroxyl group (O-H) attached to the same carbon atom. Carboxylic acids are a class of molecules which are characterized by the presence of one carboxyl group.
When deprotonated, carboxylate anions are extremely stable due to resonance. This enables carboxyl groups to be influential components of fatty acids and amino acids, which can be further reacted to generate esters, proteins, lipids, and alcohols within the body.
A carboxyl group (COOH) is a functional group consisting of a carbonyl group (C=O) with a hydroxyl group (O-H) attached to the same carbon atom. Carboxyl groups have the formula -C(=O)OH, usually written as -COOH or CO2H.
Carboxyl groups are commonly found in amino acids, fatty acids, and other biomolecules. An example of a less hydrophilic group is the carbonyl group (C=O), an uncharged but polar (contains partial positive and partial negative charges) functional group.