Hydrocarbons
The compounds made up of hydrogen and carbon only are called hydrocarbons. These are the simplest organic compounds and all other compounds are considered to be derived from them by the replacement of one or more hydrogen atoms by other atoms or groups of atoms.
The most important natural source of hydrocarbons is petroleum. There are two types of hydrocarbons:
- Saturated hydrocarbons
- Unsaturated hydrocarbons
Saturated Hydrocarbons or Alkanes:
- The hydrocarbons in which the carbon atoms are connected by only single bonds are called saturated hydrocarbons or alkanes.
- The general formula of saturated hydrocarbons are alkanes is CnH2n+2, where n is the number of carbon atoms in one molecule. The first few alkanes are methane (CH4), ethane (C2H6), and propane (C3H8).
- The saturated hydrocarbons are not very reactive.
- The saturated hydrocarbons generally give a clean flame. This is because the percentage of carbon is comparatively low which gets oxidized completely on combustion.
Structure of Saturated Hydrocarbons
The first step is to link the carbon atoms together with a single bond and then use the hydrogen atoms to satisfy the remaining valencies of carbon as shown in fig below.

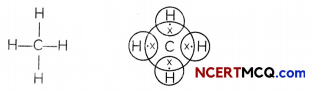
Methane: The simplest alkane is methane (CH4).
Hydrogen has a valency of 1. As carbons has four valence electrons, carbon shares these electrons with four atoms of hydrogen in order to achieve noble gas
configuration.
![]()
It is widely used as a fuel and is a major component of bio-gas and Compressed Natural Gas (CNG).
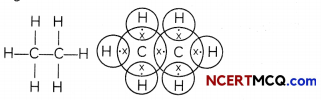
Ethane: Ethane is an alkane having two carbon atoms. The molecular formula of ethane is C2H6. There are seven single covalent bonds present in one molecule of ethane – one covalent bond between the two carbon atoms and six single bonds between carbon and hydrogen atoms.
Unsaturated Hydrocarbons (Alkenes and Alkynes)
- The hydrocarbons in which the two carbon atoms are connected by a double bond or a triple bond are called unsaturated hydrocarbons.
- Unsaturated hydrocarbons may be alkenes (CnH2n) or alkynes (CnH2n-2).
- The general formula of an alkene is CnH2n, where n is the number of carbon atoms in one molecule.
- The general formula of an alkyne is CnH2n-2, where n is the number of carbon atoms in one molecule.
- These are more reactive than saturated hydrocarbons due to the presence of double and triple bonds which are the sites of chemical reactivity.
- These give a yellow flame with lots of black smoke. This is because the percentage of carbon is comparatively higher than saturated hydrocarbons which does not oxidize completely on combustion.
Structure of Unsaturated Hydrocarbons
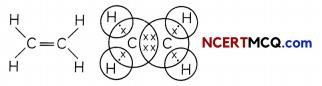
In the first step, two carbon atoms linked together by single bond. Each carbon atom combines with two hydrogen atoms. One valency per carbon atom remains unsatisfied which can be satisfied only if there is a double bond between the two carbon atoms. Ethene : The simplest alkene: Ethene is the simplest alkene having two carbon atoms and its molecular formula is C2H4. There is a double bond between the two carbon atoms and four single bonds between carbon and hydrogen atoms.
Ethyne: The simplest alkyne: The simplest alkyne is ethyne having two carbon atoms and its molecular formula is C2H2. There is a triple bond between the two carbon atoms and two single bonds between carbon and hydrogen atoms.
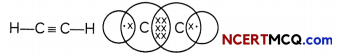
Chains, Branches and Rings
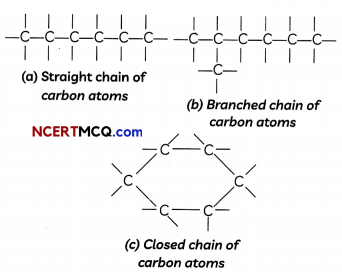
Carbon atoms can form Long chains’ containing tens of carbon atoms.
When carbon atoms combine, three types of chains can be formed:
- Straight chains
- Branched chains
- Closed chains or ring-type chains.
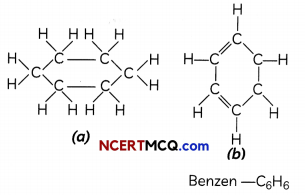
Some compounds have carbon atoms arranged in the form of a ring as in the case of cyclohexane (C6H12). Its structure is shown below. Similarly, the structure of benzene (C6H6) is also shown alongside:
![]()
Example 1.
What wilt be the formula and electron dot structure of cyclopentane?
Answer:
The formula of cyclopentane is C5H10 and its electron dot structure is given below:
Structural formula:
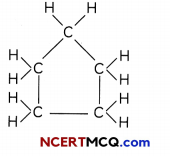
Electron dot Structure:
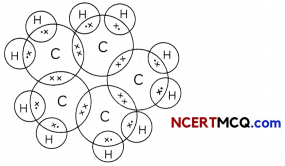
Structural Isomerism:
Organic compounds having some molecular formula but different physical and chemical properties due to different structures or catted structural isomers and this property is called isomerism. Isomerism is possible only with hydrocarbons having 4 or more carbon atoms.
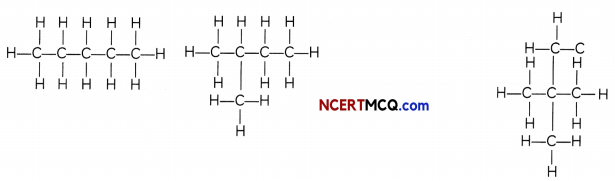
If we look at the structure of butane (C4H10), we find that two different skeletons’ are possible with four carbon atoms having a single covalent bond:
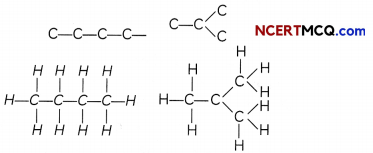
We find that both these compounds have the same molecular formula C4H10 but different structures and hence they are called Give isomers.
Isomers of hexane:
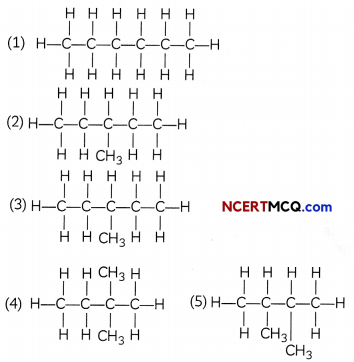
![]()
Example 2.
How many structural isomers can you draw for pentane?
Answer:
The molecular formula for pentane is C5H12. There are three structural isomers of’ pentane as given below