Strength Of Acid And Bases
The strength of acids and bases depends on the number of H+ ions and OH– ions produced, respectively.
More the number of H+ ions produced in solution, stronger is the acid and more the number of OH– ions produced in solution, stronger is the base.
If we take hydrochloric acid and acetic acid of the same concentration, say one molar, then these produce different amounts of hydrogen ions.
The pH Scale:
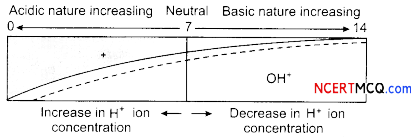
It is a scale for measuring hydrogen ion concentration in a solution. The p in pH stands for ‘potenz’ in German, meaning power. On the pH scale we can measure pH from O (very acidic) to 14 (very aLkaLine).
Higher the hydronium ion concentration, the lower is the pH value. The pH of a neutraL solution is 7.
Acidic nature increasling Neutral Basic nature increasing
Important
The solution having Lower pH will have more hydrogen ion concentration.
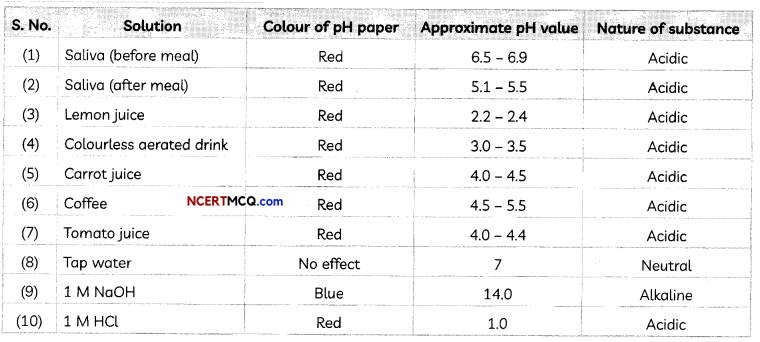
pH of some Common Substances
![]()
Example 1.
Do basic solutions also have H+(aq) ions? If yes, then why are these basic?
Answer:
Yes, basic solutions also have hydrogen ions. They are basic because in such solutions, the concentration of hydroxide ions is much greater than the concentration of hydrogen ions.
Higher the concentration of hydroxide ions, more basic is the solution.
Example 2.
Five solutions A, B, C, D and E when tested with universal indicator showed pH as 4, 1, 11, 7 and 9, respectively. Which solution is
(A) neutral?
Answer:
Solution D is neutral as it has pH value equal to 7.
(B) strongly alkaline?
Answer:
Solution C is strongly acidic as it has pH value greater than 7 and closer to 14
(C) strongly acidic?
Answer:
Solution B is strongly acidic as it has pH value less than 7 and closer to 0.
(D) weakly acidic?
Answer:
Solution A is weakly acidic as it has pH value less than 7 and closer to 7.
(E) weakly alkaline?
Arrange the pH in increasing order of hydrogen-ion concentration.
Answer:
We can arrange the solutions alon,g with their pH values as:
(A) 4
(B) 1
(C) 11
(D) 7
(E) 9
Solution E is weakly basic as it has pH value greater than 7 and closer to 7.
As pH of a solution varies inversely as the H+ ion concentration, the pH value in increasing order of H+ ion concentration is: C < E < D < A < B
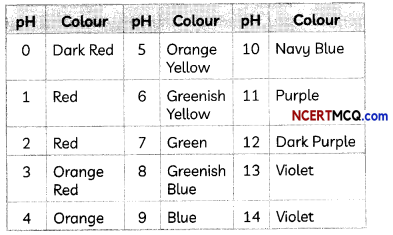
Universal Indicator:
A universal indicator is a mixture of several indicators or dyes which gives different colours at different concentrations of hydrogen ions in a solution.
The colours produced by the universal indicator at various values of pH are given below:
Importance of pH in Everyday Life:
Plants and animals are pH-sensitive: Our body works within the pH range of 7.0 to 7.8. Living organisms can survive only in a narrow range of pH change.
Acid Rain: When pH of rainwater is less than 5.6, it is called acid rain. When acid rain flows into the rivers, it lowers the pH of the river water which makes the survival of aquatic life in such rivers very difficult. pH of soil Plants require a specific pH range for their healthy growth. Farmers often treat their soil with basic substances such as quick lime (calcium oxide) or slaked lime (calcium hydroxide) if the soil is found to be acidic.
pH in our digestive system and acidity: Our stomach produces hydrochloric acid which helps in the digestion of food without harming the stomach. During indigestion the stomach produces too much acid which causes pain and irritation. People use bases called antacids to get rid of this pain by neutralizing the excess acid.
Example of antacid: Magnesium hydroxide (Milk of magnesia)
pH of milk:
The pH of fresh milk is 6, which is slightly acidic. The milkman, therefore, adds a small amount of sodium hydrogen carbonate, which is basic in nature, so as to shift the pH of fresh milk to make it slightly alkaline so as to preserve the fresh milk for longer time.
![]()
Example 3.
Fresh milk has a pH of 6. How do you think the pH will change as it turns into curd? Explain your answer.
Answer:
The pH of fresh milk will decrease from 6 as it turns into curd as lactic acid is produced during curd formation, which lowers the pH of fresh milk.
Example 4.
A milkman adds a very small amount of baking soda to fresh milk.
(A) Why does he shift the pH of the fresh milk from 6 to slightly alkaline?
Answer:
As the fresh milk has a pH of 6, it is slightly acidic. The milkman therefore adds a very small amount of a basic substance such as baking soda to fresh milk to shift the pH from 6 to slightly alkaline so that milk does not turn sour easily.
(B) Why does this milk take a long time to set as curd?
Answer:
This milk is slightly alkaline due to which it will take longer to form curd and become sour since its pH value is now greater than 7.
pH change as the cause of tooth decay: Tooth decay starts when the pH of the mouth is lower than 5.5. Tooth enamel, made up of calcium phosphate is the hardest substance in the body. Bacteria present in the mouth produce acids by degradation of sugar and food particles remaining in the mouth after eating. Using toothpastes, which are generally basic, for cleaning the teeth can neutralise the excess acid and prevent tooth decay.
Self-defence by animals and plants through chemical warfare: Bee-sting leaves an acid which causes pain and irritation. Use of a mild base like baking soda on the stung area gives relief. Stinging hair of nettle leaves inject methanoic acid causing burning pain.
pH of plants: The leaves of Nettle, which is a herbaceous plant that grows in the wild, have stinging hair, which cause painful stings when touched accidentallydue to the methanoic acid secreted by them. However, nature provides neutralization options in the form of dock plant, which often grows beside the nettle in the wild, whose leaf is rubbed on the stung area to provide relief from pain and irritation.
Example 5.
Under what soil condition do you think a farmer would treat the soil of his fields with quick lime (calcium oxide) or slaked lime (calcium hydroxide) or chalk (calcium carbonate)?
Answer:
A farmer would treat the soil of his fields with quicklime or slaked lime or chalk if the soil has become acidic as all these fertilizers are basic. This will neutralize the acidic nature of the soil.