Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Test for Aldehydes
(i) Tollens Reagent Test
Tollens reagent is an ammonical silver nitrate solution. When an aldehyde is warmed with Tollens reagent a bright silver mirror is produced due to the formation of silver metal. This reaction is also called silver mirror test for aldehydes.
![]()
(ii) Fehlings Solution Test
Fehlings solution is prepared by mixing equal volumes of Fehlings solution ‘A’ containing aqueous copper sulphate and Fehlings solution ‘B’ containing alkaline solution of sodium potassium tartarate (Rochelle salt)
When aldehyde is warmed with Fehlings solution deep blue colour solution is changed to red precipitate of cuprous oxide.
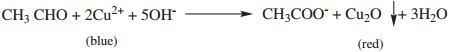
(iii) Benedict’s Solution Test:
Benedicts solution is a mixture of CuSO4 + sodium citrate + NaOH.Cu2+ is reduced by aldehyde to give red
precipitate of cuprous oxide.
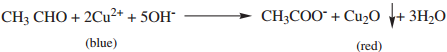
(iv) Schiff’ Reagent Test
Dilute solution of aldehydes when added to schiff’ reagent (Rosaniline hydrochloride dissolved in water and its red colour decolourised by passing SO2) yields its red colour. This is known as Schiff’ test for aldehydes. Ketones do not give this test. Acetone however gives a positive test but slowly.
An aldehyde is similar to a ketone, except that instead of two side groups connected to the carbonyl carbon, they have at least one hydrogen (RCOH). The simplest aldehyde is formaldehyde (HCOH), as it has two hydrogens connected to the carbonyl group.
Tollen’s reagent is a classical organic laboratory technique to test for the presence of an aldehyde. The reagent consists of silver (I) ions dissolved in dilute ammonia. When the aldehyde is oxidized, the silver (I) ions are reduced to silver metal.
The Schiff test is a chemical test used to check for the presence of aldehydes in a given analyte. This is done by reacting the analyte with a small quantity of a Schiff reagent (which is the product formed in certain dye formulation reactions such as the reaction between sodium bisulfite and fuchsin).
Aldehyde, any of a class of organic compounds in which a carbon atom shares a double bond with an oxygen atom, a single bond with a hydrogen atom, and a single bond with another atom or group of atoms (designated R in general chemical formulas and structure diagrams).
Fehling’s solution can be used to distinguish aldehyde vs ketone functional groups. The compound to be tested is added to the Fehling’s solution and the mixture is heated. Aldehydes are oxidized, giving a positive result, but ketones do not react, unless they are α-hydroxy ketones.
Take the given organic compound in a clean test tube. Add 1ml of chromic acid reagent to the given organic compound. The appearance of a green or blue colour precipitate indicates the presence of aldehydes.