Online Education HOTS Questions for Class 9 Science Chapter 4 Structure of the Atom
These Solutions are part of Online Education HOTS Questions for Class 9 Science. Here we have given HOTS Questions for Class 9 Science Chapter 4 Structure of the Atom
Question 1.
Both helium (He) and beryllium (Be) have two valence electrons. Whereas He represents a noble gas element, Be does not. Assign reason.
Answer:
The element He (Z = 2) has two electrons present in the only shell i.e., K-shell. Since this shell can have a maximum of two electrons only therefore, He is a noble gas element. The element Be (Z = 4) has electronic configuration as : 2, 2. Although the second shell has also two electrons but it is not complete. It can still accommodate six more electrons. Therefore, the element beryllium does not represent a noble gas element.
More Resources
- HOTS Questions for Class 9 Science
- NCERT Solutions for Class 9 Science
- Value Based Questions in Science for Class 9
- NCERT Exemplar Solutions for Class 9 Science
- Previous Year Question Papers for CBSE Class 9 Science
Question 2.
Study the data given below and answer the questions which follow :
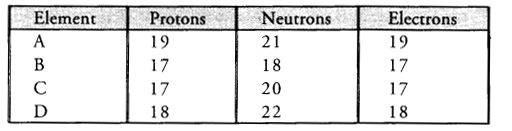
- Write the mass number and atomic number of the particles A, B, C and D.
- Which represent a pair of isotopes ?
Answer:
- Particle A : Mass number = 7 ; Atomic number = 3
Particle B : Mass number = 17 ; Atomic number = 9
Particle C : Mass number =16, Atomic number = 8
Particle D : Mass number =18, Atomic number = 8 - Particles C and D represent a pair of isotopes since they have same atomic number.
Question 3.
Which of the two will be chemically more reactive ; element X with atomic number 17 or element Y with atomic number 16 ?
Answer:
The electronic configuration of the two elements are as follows :
X(Z = 16): K (2), L(8), M(6) ;
Y(Z = 17): K(2), L(8), M(7)
The element X needs two electrons in the M shell to have the noble gas configuration of element, Ar (Z = 18). However, the element Y needs only one electron to achieve this. This means that the element Y has a greater urge or desire to take up one electron from an outside atom. It is therefore, more reactive than the element X which needs two electrons.
Question 4.
The number of protons, neutrons and electrons in particles from A to E are given below :
- Which one is a cation ?
- Which one is an anion ?
- Which represent pair of isotopes ?
Answer:
- B is a monovalent cation (B+)
- E is a monovalent anion (E–)
- A and D represent pair of isotopes.
Question 5.
An atom of an element has three electrons in the third shell which is the outermost shell. Write
- the electronic configuration
- the atomic number
- number of protons
- valency
- the name of the element
- its nature whether metal or non-metal. (CBSE 2012)
Answer:
The third shell is M shell. If the atom of the element has three electrons in the third shell, this means that K and L shells are already filled.
- Electronic configuration : 2, 8, 3.
- Atomic number = No. of electrons =13
- Number of protons = No. of electrons =13
- Valency of the element = 3
- The element with Z = 13 is aluminium (Al)
- It is a metal.
Hope given HOTS Questions for Class 9 Science Chapter 4 Structure of the Atom are helpful to complete your science homework.
If you have any doubts, please comment below. Learn Insta try to provide online science tutoring for you.