Check the below Online Education NCERT MCQ Questions for Class 9 Science Chapter 4 Structure of the Atom with Answers Pdf free download. MCQ Questions for Class 9 Science with Answers were prepared based on the latest exam pattern. We have Provided Structure of the Atom Class 9 Science MCQs Questions with Answers to help students understand the concept very well. https://ncertmcq.com/mcq-questions-for-class-9-science-with-answers/
You can refer to NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom to revise the concepts in the syllabus effectively and improve your chances of securing high marks in your board exams.
Class 9 Science Chemistry Chapter 4 MCQ With Answers
Chemistry Class 9 Chapter 4 MCQs On Structure of the Atom
Structure Of Atom Class 9 MCQ Question 1.
Which of the following correctly represents the electronic distribution in the Mg atom?
(a) 3, 8, 1
(b) 2, 8, 2
(c) 1, 8, 3
(d) 8, 2, 2
Answer
Answer: (b) 2, 8, 2
Structure Of Atom Class 9 MCQ With Answers Question 2.
Rutherford’s ‘alpha (α) particles scattering experiment’ resulted in the discovery of
(a) electron
(b) proton
(c) nucleus in the atom
(d) atomic mass
Answer
Answer: (c) nucleus in the atom
Class 9 Science Chapter 4 MCQ Question 3.
The number of electrons in an element X is 15 and the number of neutrons is 16. Which of the following is the correct representation of the element?
(a) \(_{15}^{31}\)X
(b) \(_{16}^{31}\)X
(c) \(_{15}^{16}\)X
(d) \(_{16}^{15}\)X
Answer
Answer: (a) \(_{15}^{31}\)X
MCQ On Structure Of Atom Class 9 Question 4.
Which of the following are true for an element?
(i) Atomic number = number of protons + number of electrons
(ii) Mass number = number of protons + number of neutrons
(iii) Atomic mass = number of protons = number of neutrons
(iv) Atomic number = number of protons = number of electrons
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (ii) and (iv)
Answer
Answer: (d) (ii) and (iv)
Structure Of Atom MCQ Class 9 Question 5.
Atomic models have been improved over the years. Arrange the following atomic models in the order of their chronological order
(i) Rutherford’s atomic model
(ii) Thomson’s atomic model
(ii) Bohr’s atomic model
(a) (i), (ii) and (iii)
(b) (ii), (iii) and (i)
(c) (ii), (i) and (iii)
(d) (iii), (ii) and (i)
Answer
Answer: (c) (ii), (i) and (iii)
Class 9 Science Chapter 4 MCQ With Answers Question 6.
The ion of an element has 3 positive charges. Mass number of the atom is 27 and the number of neutrons is 14. What is the number of electrons in the ion?
(a) 13
(b) 10
(c) 14
(d) 16
Answer
Answer: (b) 10
Class 9 Science Ch 4 MCQ Question 7.
Identify the Mg2+ ion from the figure where, n and p represent the number of neutrons and protons respectively.
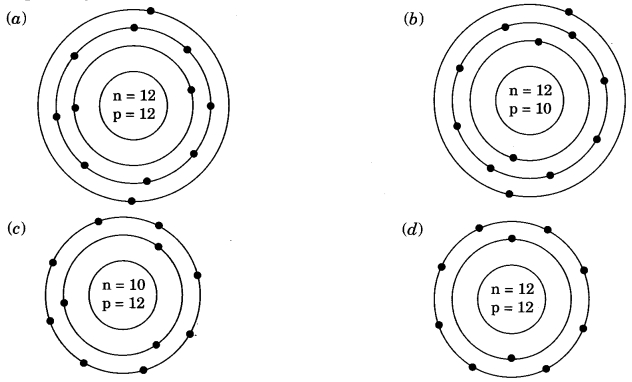
Answer
Answer: (d)
MCQ Structure Of Atom Class 9 Question 8.
The first model of an atom was given by
(a) N. Bohr
(b) E. Goldstein
(c) Rutherford
(d) J.J. Thomson
Answer
Answer: (d) J.J. Thomson

Structure Of The Atom Class 9 MCQ Question 9.
An atom with 3 protons and 4 neutrons will have a valency of
(a) 3
(b) 7
(c) 1
(d) 4
Answer
Answer: (c) 1
Class 9 Structure Of Atom MCQ Question 10.
Which of the following statement is always correct?
(a) An atom has equal number of electrons and protons.
(b) An atom has equal number of electrons and neutrons.
(c) An atom has equal number of protons and neutrons.
(d) An atom has equal number of electrons, protons and neutrons.
Answer
Answer: (a) An atom has equal number of electrons and protons.
MCQ On Structure Of Atom Class 9 Question 11.
Which of the following statements about Rutherford’s model of atom are correct?
(i) Considered the nucleus as positively charged.
(ii) Established that the a-particles are four times as heavy as a hydrogen atom.
(iii) Can be compared to solar system.
(iv) Was in agreement with Thomson’s model.
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) only (i)
Answer
Answer: (a) (i) and (iii)
MCQ Questions For Class 9 Science Chapter 4 Question 12.
Hydrogen exists in three isotopic forms, \(_{1}^{1}\)H, \(_{1}^{2}\)H, \(_{1}^{3}\)H known as protium, deuterium and tritium. Why are all the isotopes neutral in nature?
(a) Since neutrons are neutral in nature hence isotopes are electrically neutral.
(b) All the isotopes have one electron and one proton, hence they are neutral.
(c) All the isotopes have one proton and one neutron, hence they are neutral.
(d) Increasing number of protons in the isotopes make them neutral.
Answer
Answer: (b) All the isotopes have one electron and one proton, hence they are neutral.
Ch 4 Science Class 9 MCQ Question 13.
How many electrons are present in M-shell of an element with atomic number 20?
(a) 5
(b) 8
(c) 12
(d) 18
Answer
Answer: (b) 8
Chapter 4 Science Class 9 MCQ Question 14.
There are two atomic species X and Y, such that

Which of the following statements is true about X and Y?
(a) X and Y are isobars.
(b) X and Y have different chemical properties.
(c) X and Y have different physical properties.
(d) All of these.
Answer
Answer: (c) X and Y have different physical properties.
MCQ Of Structure Of Atom Class 9 Question 15.
The ion of an element has 3 positive charges. Mass number of the atom is 27 and the number of neutrons is 14. What is the number of electrons in the ion?
(a) 13
(b) 10
(c) 14
(d) 16
Answer
Answer: (b) 10
Question 16.
An alpha particle is also known as:
(a) subatomic particle
(b) an unionised helium atom
(c) a neutral particle
(d) a doubly-charged helium ion
Answer
Answer: (d) a doubly-charged helium ion
Question 17.
There are three isotopes of carbon which are named as C-12, C-13 and C-14 out of which C-12 is the most abundant isotope. In the given structures of 3 isotopes, what will be the composition of the nucleus?
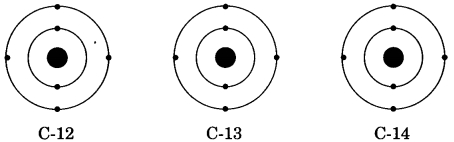
(a) C-12 : 6p + 6n, C-13 : 7p + 6n, C-14 : 8p + 6n
(b) C-12 : 6p + 6n, C-13 : 6p + 7n, C-14 : 6p + 8n
(c) C-12 : 6p + 6n, C-13 : 5p + 8n, C-14 : 7p + 7n
(d) C-12 : 6p + 6n, C-13 : 12p + 1n, C-14 : 5p + 9n
Answer
Answer: (b) C-12 : 6p + 6n, C-13 : 6p + 7n, C-14 : 6p + 8n
Question 18.
The charge on an electron is equal to:
(a) 1.6 × 10-19 C of -ve charge
(b) 2.6 × 10-19 C of -ve charge
(c) 1.6 × 10-22 C of -ve charge
(d) 1.6 × 10-23 C of -ve charge
Answer
Answer: (a) 1.6 × 10-19 C of -ve charge
Question 19.
Which of the following isotope is used in the treatment of blood cancer?
(a) P-32
(b) I-131
(c) Co-60
(d) any of these
Answer
Answer: (a) P-32
Question 20.
Isobars do not differ in the number of
(a) protons
(b) electrons
(c) neutrons
(d) nucleons
Answer
Answer: (d) nucleons

Fill in the blanks
1. Rutherford’s a-particle scattering experiment led to the discovery of the ……………….
Answer
Answer: atomic nucleus
2. Isotopes have same ……………… but different ……………..
Answer
Answer: atomic number, mass number
3. Neon and chlorine have atomic numbers 10 and 17 respectively. Their valencies will be ………….. and ……………. respectively.
Answer
Answer: 0 and 1
4. The electronic configuration of silicon is …………… and that of sulphur is ……………..
Answer
Answer: 2, 8, 4, and 2, 8, 6
5. Cathode rays consist of negatively charged particles known as ………………
Answer
Answer: electrons
6. ……………. are the atoms containing same number of neutrons.
Answer
Answer: isotones
7. The outermost shell of an atom is known as its ……………..
Answer
Answer: valence shell
8. Alpha particles are helium nuclei (He2+) and have …………. mass and ……………. units charge.
Answer
Answer: 4u and +2
9. The maximum number of electrons in a shell is given by ……………..
Answer
Answer: 2n²
10. Neutrons were discovered by …………….
Answer
Answer: James Chadwick
Match the names of the scientists given the columns A with their contributions towards the understanding of the atomic structure as given in column B.
1.
| Column A | Column B |
| (a) Ernest Rutherford | (i) Indivisibility of atoms |
| (b) J. J. Thomson | (ii) Stationary orbits |
| (c) Dalton | (iii) Concept of nucleus |
| (d) Neils Bohr | (iv) Discovery of electrons |
| (e) James Chadwick | (v) Atomic number |
| (f) E. Goldstein | (vi) Neutron |
| (g) Mosley | (vii) Canal rays |
Answer
Answer:
| Column A | Column B |
| (a) Ernest Rutherford | (iii) Concept of nucleus |
| (b) J. J. Thomson | (iv) Discovery of electrons |
| (c) Dalton | (i) Indivisibility of atoms |
| (d) Neils Bohr | (ii) Stationary orbits |
| (e) James Chadwick | (vi) Neutron |
| (f) E. Goldstein | (vii) Canal rays |
| (g) Mosley | (v) Atomic number |
2.
| Column A | Column B |
| (a) Ions | (i) Same mass number, different atomic numbers. |
| (b) Isobars | (ii) Same number of neutrons, different mass numbers. |
| (c) Isotopes | (iii) Formed by loss or gain of electron |
| (d) Isotones | (iv) Combining capacity of an atom. |
| (e) Valency | (v) Same atomic numbers, different mass numbers. |
Answer
Answer:
| Column A | Column B |
| (a) Ions | (iii) Formed by loss or gain of electron |
| (b) Isobars | (i) Same mass number, different atomic numbers. |
| (c) Isotopes | (v) Same atomic numbers, different mass numbers. |
| (d) Isotones | (ii) Same number of neutrons, different mass numbers. |
| (e) Valency | (iv) Combining capacity of an atom. |
3.
| Column A | Column B |
| Chlorine atom | 2, 8 |
| Sodium atom | 2, 8, 7 |
| Magnesium ion | 2, 8, 2 |
| Magnesium atom | 2, 8, 1 |
Answer
Answer:
| Column A | Column B |
| Chlorine atom | 2, 8 |
| Sodium atom | 2, 8, 7 |
| Magnesium ion | 2, 8, 2 |
| Magnesium atom | 2, 8, 1 |
Answer the questions in one word:
1. Which symbols are used to represent different Bohr’s orbits?
Answer
Answer: K, L, M, N
2. Which isotope is used in the treatment of cancer?
Answer
Answer: Cobalt-60
3. What is the charge of anode rays?
Answer
Answer: positive
4. There are 14 protons and 13 neutrons in the nucleus of an atom. What is its mass number?
Answer
Answer: 27
5. Indicate the number of electrons, protons and neutrons in the element \(_{19}^{39}\)K.
Answer
Answer: e = 19, p = 19, n = 20
6. The atomic number of cation M2+ is 12. How many electrons are present in it?
Answer
Answer: 10
7. Which isotope of hydrogen is called protium?
Answer
Answer: \(_{1}^{1}\)H
8. Which particles determine the mass of an atom?
Answer
Answer: Protons, neutrons
Solve the crossword with the help of clues:
Across:
1. Part of atom responsible for mass.
3. Same mass number of species.
5. a-particle scattering experiment.
7. No. of valence electron in sodium.
Down:
2. Discovered the neutron.
4. Latest atomic model given by.
6. Negatively charged subatomic particle.
8. Positively charged substance particle.
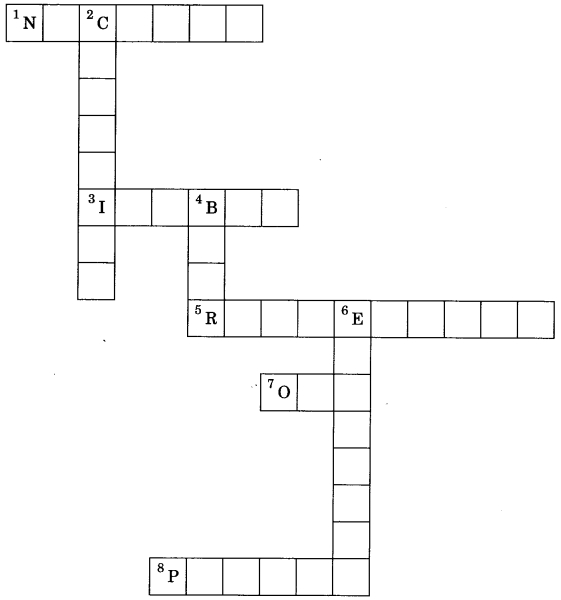
Answer
Answer:
Across:
1. Nucleus
3. Isobar
5. Rutherford
7. One
Down:
2. Chadwick
4. Bohr
6. Electron
8. Proton
We hope the given NCERT MCQ Questions for Class 9 Science Chapter 4 Structure of the Atom with Answers Pdf free download will help you. If you have any queries regarding Structure of the Atom CBSE Class 9 Science MCQs Multiple Choice Questions with Answers, drop a comment below and we will get back to you soon.
Class 9 Science Chemistry MCQ: