Check the below Online Education NCERT MCQ Questions for Class 9 Science Chapter 3 Atoms and Molecules with Answers Pdf free download. MCQ Questions for Class 9 Science with Answers were prepared based on the latest exam pattern. We have Provided Atoms and Molecules Class 9 Science MCQs Questions with Answers to help students understand the concept very well. https://ncertmcq.com/mcq-questions-for-class-9-science-with-answers/
You can refer to NCERT Solutions for Class 9 Science Chapter 3 Atoms and Molecules to revise the concepts in the syllabus effectively and improve your chances of securing high marks in your board exams.
Class 9 Science Chemistry Chapter 3 MCQ With Answers
Chemistry Class 9 Chapter 3 MCQs On Atoms and Molecules
Atoms And Molecules Class 9 MCQ Question 1.
The chemical symbol for nitrogen gas is
(a) Ni
(b) N2
(c) N+
(d) N
Answer
Answer: (b) N2
Atoms And Molecules Class 9 MCQ With Answers Question 2.
The chemical symbol for sodium is
(a) So
(b) Sd
(c) NA
(d) Na
Answer
Answer: (d) Na
Converting grams to moles is super easy if you use mass to moles calculator above.
Class 9 Science Chapter 3 MCQ Question 3.
Which of the following would weigh the highest?
(a) 0.2 mole of sucrose (C12H22O11)
(b) 2 moles of CO2
(c) 2 moles of CaCO3
(d) 10 moles of H2O
Answer
Answer: (c) 2 moles of CaCO3
Class 9 Atoms And Molecules MCQ Question 4.
Which of the following has maximum number of atoms?
(a) 18 g of H2O
(b) 18 g of O2
(c) 18 g of CO2
(d) 18 g of CO4
Answer
Answer: (d) 18 g of CO4

Atom And Molecules Class 9 MCQ Question 5.
Which of the following correctly represents 360 g of water?
(i) 2 moles of H2O
(ii) 20 moles of water
(iii) 6.022 × 1023 molecules of water
(iv) 1.2044 × 1025 molecules of water
(a) (i)
(b) (i) and (iv)
(c) (ii) and (iii)
(d) (ii) and (iv)
Answer
Answer: (d) (ii) and (iv)
Heat & Mass Transfer MCQ with detailed explanation for interview, entrance and competitive exams.
MCQ On Atoms And Molecules Class 9 Question 6.
3.42 g of sucrose are dissolved in 18 g of water in a beaker. The number of oxygen atoms in the solution are
(a) 6.68 × 1023
(b) 6.09 × 1022
(c) 6.022 × 1023
(d) 6.022 × 102321
Answer
Answer: (a) 6.68 × 1023
MCQ Of Atoms And Molecules Of Class 9 Question 7.
A change in the physical state can be brought about
(a) only when energy is given to the system
(b) only when energy is taken out from the system
(c) when energy is either given to, or taken out from the system
(d) without any energy change
Answer
Answer: (c) when energy is either given to, or taken out from the system
Class 9 Science Ch 3 MCQ Question 8.
The relative molecular mass of Na2S2O3.5H2O is
(a) 250 amu
(b) 250 g
(c) 248 amu
(d) 248 g
Answer
Answer: (c) 248 amu
MCQ Atoms And Molecules Class 9 Question 9.
Which of the following has maximum number of atom?
(a) 18 g H2O
(b) 18 g of O2
(c) 18 g of CO2
(d) 18 g of CH4
Answer
Answer: (d) 18 g of CH4
Ch 3 Science Class 9 MCQ Question 10.
Percentage of calcium in calcium carbonate is
(a) 40
(b) 30
(c) 48
(d) 36
Answer
Answer: (a) 40
Atoms And Molecules MCQ Class 9 Question 11.
Which has maximum number of molecules?
(a) 1 g of CO2
(b) 1 g of N2
(c) 1 g of H2
(d 1 g of CH4
Answer
Answer: (c) 1 g of H2
Class 9 Chemistry Chapter 3 MCQ Question 12.
What mass of carbon dioxide (CO2) will contain 3.011 × 1023 molecules?
(a) 11.0 g
(b) 22.0 g
(c) 4.4 g
(d) 44.0 g
Answer
Answer: (b) 22.0 g
Class 9 Chapter 3 Science MCQ Question 13.
The value of Avogadro’s constant is:
(a) 6.0 × 1024
(b) 6.01 × 1022
(c) 6.022 × 1023
(d) 6.022 × 10-23
Answer
Answer: (c) 6.022 × 1023
Class 9 Science Chapter 3 MCQ With Answers Question 14.
How many times an atom of sulphur is heavier than an atom of carbon?
(a) 32 times
(b) 12 times
(c) 8/3 times
(d) 12/32 times
Answer
Answer: (c) 8/3 times
Chapter 3 Science Class 9 MCQ Question 15.
The number of oxygen atoms in 4.4 g of CO2 is approx.
(a) 6 × 1022
(b) 6
(c) 12 × 1023
(d) 1.2 × 1023
Answer
Answer: (d) 1.2 × 1023
Question 16.
Which of the following represents 12 u?
(a) Mass of 1 hydrogen atom
(b) Mass of C-12 atom
(c) Mass of 0-16 atom
(d) 1/12th of mass of C-12 atom.
Answer
Answer: (d) 1/12th of mass of C-12 atom.
Question 17.
Which of the following would weigh the highest?
(a) 0.2 mole of sucrose (C12H22O11)
(b) 2 moles of CO2
(c) 2 moles of CaCO3
(d) 10 moles of H2O
Answer
Answer: (c) 2 moles of CaCO3
Question 18.
The molecule having an atomicity of 4 is:
(a) Sulphate molecule
(b) Ozone molecule
(c) Phosphorus molecule
(d) Methane molecule
Answer
Answer: (c) Phosphorus molecule

Fill in the blanks
1. In a chemical reaction, the sum of the masses of the reactants and products remain unchanged. This is called ……………
Answer
Answer: Law of Conservation of Mass
2. A group of atoms carrying a fixed charge on them is called …………..
Answer
Answer: Polyatomic ion
3. The formula unit mass of Ca3(PO4)2 is ……………
Answer
Answer: (3 × atomic mass of calcium) + (2 × atomic mass of phosphorus) + (8 × atomic mass of oxygen) = 310
4. Formula of sodium carbonate is ………….. and that of ammonium sulphate is ……………
Answer
Answer: Na2CO3 (NH4)2 SO4
5. Elements are represented by symbols and molecules by …………….
Answer
Answer: Chemical formula
6. The combining power of an element is known as its …………….
Answer
Answer: Valency
7. An atom or a group of atoms which carries positive or negative charge is called an ……………..
Answer
Answer: ion
8. The volume occupied by one mole of a gas under standard conditions of temperature and pressure is called ……………..
Answer
Answer: molar volume
9. The number of atoms present in one molecule of the substance is called its ……………
Answer
Answer: atomicity
10. Atomicity of H2SO4 is …………….
Answer
Answer: 7
Match the following columns
1.
| Column I | Column II |
| (a) 28 g of He | (i) 58.54 |
| (b) 0.5 mole of O2 | (ii) 7 mol |
| (c) Molecular mass of common salt | (iii) 60 g |
| (d) 1.5 mole of Ca | (iv) 16 g |
Answer
Answer:
| Column I | Column II |
| (a) 28 g of He | (ii) 7 mol |
| (b) 0.5 mole of O2 | (iv) 16 g |
| (c) Molecular mass of common salt | (i) 58.54 |
| (d) 1.5 mole of Ca | (iii) 60 g |
2.
| Column I | Column II |
| (a) Sodium carbonate | (i) NaHCO3 |
| (b) Sodium bicarbonate | (ii) Na3PO4 |
| (c) Sodium sulphate | (iii) Na2CO3 |
| (d) Sodium phosphate | (iv) Na2SO4 |
Answer
Answer:
| Column I | Column II |
| (a) Sodium carbonate | (iii) Na2CO3 |
| (b) Sodium bicarbonate | (i) NaHCO3 |
| (c) Sodium sulphate | (iv) Na2SO4 |
| (d) Sodium phosphate | (ii) Na3PO4 |
3.
| Column I | Column II |
| (a) Silver | (i) Na |
| (b) Sodium | (ii) Ag |
| (c) Gold | (iii) K |
| (d) Potassium | (iv) Au |
Answer
Answer:
| Column I | Column II |
| (a) Silver | (ii) Ag |
| (b) Sodium | (i) Na |
| (c) Gold | (iv) Au |
| (d) Potassium | (iii) K |
Answer the questions in one word:
1. Smallest particle which cannot be divided into simpler parts.
Answer
Answer: Parmanu (Atom)
2. Which experiment led to the discovery of the atomic nucleus?
Answer
Answer: Rutherford’s alpha particle scattering experiment
3. What is the mass of a proton and a neutron?
Answer
Answer: 1 amu
4. What do the species \(_{1}^{3}\)A and \(_{2}^{3}\)B represent?
Answer
Answer: Isobars
5. What is the formula of hydrogen carbonate?
Answer
Answer: HCO3–
6. What is the chemical formula of aluminium sulphate?
Answer
Answer: Al2(SO4)3
7. What is the formula unit mass of CaCl2?
Answer
Answer: 111 u
8. What is the mass of 5 moles H2O?
Answer
Answer: 90 g
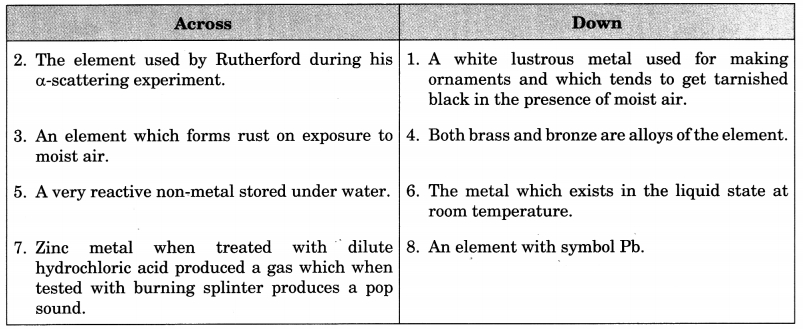
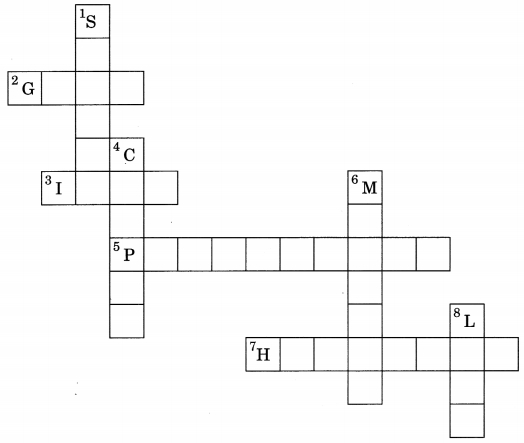
1. Complete the following crossword puzzle by using the name of the chemical elements. Use the data given in table mentioned below.
Answer
Answer:
Down:
2. Gold
3. Iron
5. Phosphorus
7. Hydrogen
Across:
1. Silver
4. Copper
6. Mercury
8. Lead
We hope the given NCERT MCQ Questions for Class 9 Science Chapter 3 Atoms and Molecules with Answers Pdf free download will help you. If you have any queries regarding Atoms and Molecules CBSE Class 9 Science MCQs Multiple Choice Questions with Answers, drop a comment below and we will get back to you soon.
Class 9 Science Chemistry MCQ:
- Matter in Our Surroundings Class 9 MCQ
- Is Matter Around Us Pure Class 9 MCQ
- Atoms and Molecules Class 9 MCQ
- Structure of the Atom Class 9 MCQ