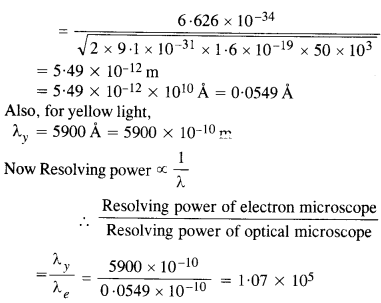
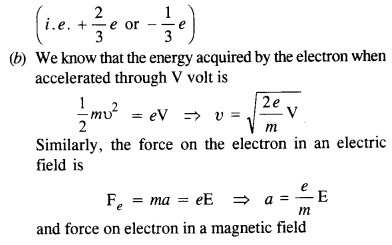
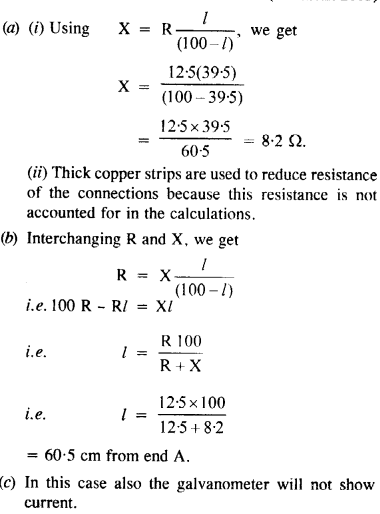
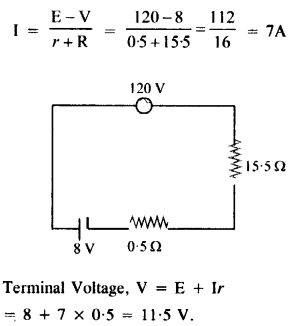
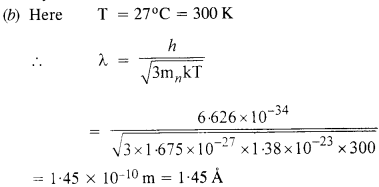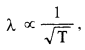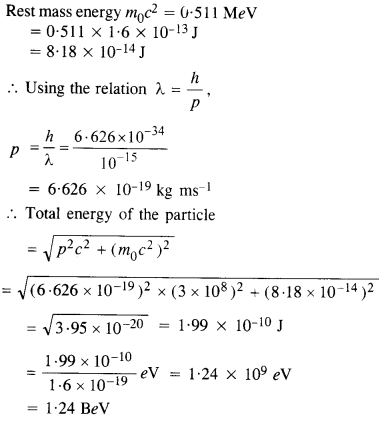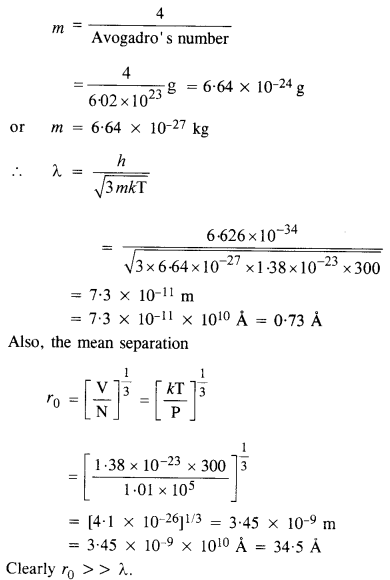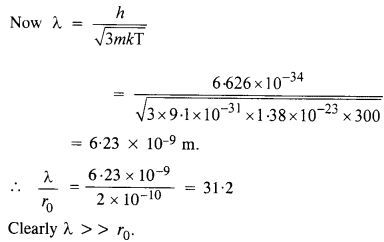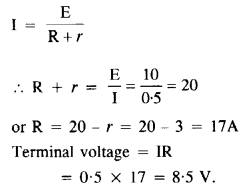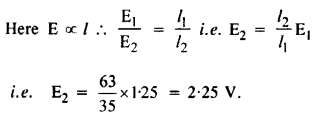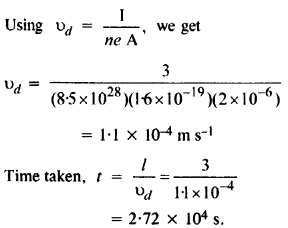NCERT Solutions for Class 12 History Chapter 6 Bhakti-Sufi Traditions Changes in Religious Beliefs and Devotional Texts are part of NCERT Solutions for Class 12 History. Here we have given NCERT Solutions for Class 12 History Chapter 6 Bhakti-Sufi Traditions Changes in Religious Beliefs and Devotional Texts.
| Board | CBSE |
| Textbook | NCERT |
| Class | Class 12 |
| Subject | History |
| Chapter | Chapter 6 |
| Chapter Name | Bhakti-Sufi Traditions Changes in Religious Beliefs and Devotional Texts |
| Number of Questions Solved | 9 |
| Category | NCERT Solutions |
NCERT Solutions for Class 12 History Chapter 6 Bhakti-Sufi Traditions Changes in Religious Beliefs and Devotional Texts
Question 1.
Explain with examples what historians mean by the integration of cults.
Solution :
The integration of cults means that there was composition, compilation and preservation of Puranic texts in simple Sanskrit verse, explicitly meant to be accessible to women and Shudras, who were generally excluded from Vedic learning. The Brahmanas also accepted and reworked the beliefs and practices of these and other social categories. The example of this integration of cults is at Puri, Orissa where the principal deity was identified as Jagannatha (literally, the lord of the world), a form of Vishnu. Here the deity is represented in a very different way. In this case, a local deity, whose image was and continues to be made of wood by local tribal specialists, was recognised as a form of Vishnu. At the same time, Vishnu was visualised in a way, that was very different from that in other parts of the country. Such instances of integration were evident amongst goddess cults as well where the local deities were provided an identity as the wife of the principal male deities – Lakshmi, the wife of Vishnu or Parvati, the wife of Shiva.
Question 2.
To what extent do you think the architecture of mosques in the subcontinent reflects a combination of universal ideals and local traditions?
Solution :
With the arrival of Islam in the Medieval ages, the architecture of Islam also came to India. However, the Arab-cum-Islamic architecture got impacted by the local traditions and rites too. Hence, we see a fusion of the two. This can be further elaborated by the examples of architecture mainly the constructions of the mosques of those days.
Some features of the architecture of mosques are universal. All mosques have orientation towards Mecca. This is manifested in the placement of Mehrab and Minar within a mosque. But at the same time we have influences that can be described only as local influences. A 13th Century mosque in Kerala has a shikhar like roof unlike a normal mosque where it is dome. The Shah Hamdan Mosque in Kashmir is made of Kashmiri woods and its facade is like that of a temple. The Atia Mosque in Bangladesh is made of bricks, though its roof is round. Thus, we can see that the architecture of Mosques is that of fusion.
Question 3.
What were the similarities and differences between the be-shari‘a and ba- shari‘a sufi traditions ?
Solution :
(a) Similarities :
- Both be-sharia and ba-shari‘a sufis turned to asceticism and mysticism and were against the growing materialism of the Caliphate as a religious and political institution.
- They are critical of the dogmatic definitions and scholastic methods of interpreting the Quran and sunna adopted by the theologians.
- Both laid emphasis on seeking salvation through intense devotion and love for God.
- Both sought an interpretation of the Quran on the basis of their personal experience.
(b) Differences :
| Be-Shari’s | Ba-Shari’s |
| (i) They scorned the Khanqah and took to mendicancy and observed Celibacy. | (i) They organised communities around the khanqah controlled by a teaching master known as Shaikh, Pir or Murshid. |
| (ii) They ignored rituals and observed extreme forms of asceticism. | (ii) They observed special rituals of initiation. The initiates took an oath of allegiance, wore a patched garment and shaved their hair. Khanqah was the centre of social life. |
| (iii) They were known by different names as Qalandars, Madaris, Malangs, and Haidaris. | (iii) The Chishti silsila is the most important and influential tradition. |
| (iv) They deliberately defied the shari‘a i.e., law governing the Muslim community. So they were called as be-shari‘a. | (iv) They complied with the sharia. |
Question 4.
Discuss the ways in which the Alvars, Nayanars and Virashaivas expressed critiques of the caste system.
Solution :
The Alvars, Nayanars and Virashaivas expressed critiques of the caste system in the following ways :
(a) Alvars and Nayanars :
- The Alvars and Nayanars protested against the caste system or at least attempted to reform the system. Their bhaktas hailed from diverse social backgrounds ranging from Brahmanas to artisans and cultivators and even from castes considered “untouchable”. Thus, people from all walks of society were welcomed by them.
- They stated that their compositions were as important as the Vedas. For example, one of the major anthologies of compositions by the Alvars, the Nalayira Divyaprabandham, was described as the Tamil Veda.
- Tondaradippodi, a Brahmana Alvar, opposed the cast system in the following way :
“You (Vishnu) manifestly like those “servants” who express their love for your feet, though
they may be bom outcastes, more than the Chaturvedins who are strangers and without allegiance to your service.” - Another saint, a Nayanar, composed the following verse in protest of the caste system : “O rogues who quote the law books, Of what use are your gotra and kula, Just bow to Marperu’s lord (Shiva who resides in Marperu, in Thanjavur, Tamil Nadu) as your sole refuge.”
(b) Virashaiva:
- Virashaiva or Lingayats challenged the idea of caste and the “pollution” attributed to certain groups by Brahmanas. As a result of it, marginalised people within the Brahmanical order joined this tradition.
- They questioned the theory of rebirth.
- The Lingayats encouraged certain practices disapproved in the Dharmashastras, such as post-puberty marriage and remarriage of widows.
Question 5.
Describe the major teachings of either Kabir or Baba Guru Nanak, and the ways in which these have been transmitted.
Solution :
- The major teachings of Kabir and Baba Guru Nanak are as given below :
- Teachings of Kabir :
- He described the Ultimate Reality as Allah, Khuda, Hazrat and Pir. He also used terms drawn on Vedantic traditions, alakh (the unseen), nirakar (formless), Brahman and Atman. Terms such as shabda (sound) or shunya (emptiness) were drawn from yogic traditions.
- There is only one God. He was against the distinction made between gods of different communities.
- Kabir was against idol worship and Hindu polytheism.
- He was in favour of the Hindu practice of nam-simaran (remembrance of God’s name).
- Teachings of Guru Nanak : The message of Baba Guru Nanak is spelt out in his hymns and teachings which are as given below :
- Baba Guru Nanak believed in nirguna bhakti.
- He was against the external practices of religions.
- He rejected sacrifices, ritual baths, image worship, austerities and the scriptures of both Hindus and Muslims.
- For him, the Absolute or “arab” had no gender or form.
- He favoured a simple way to connect to the Divine by remembering and repeating the Divine Name.
- Teachings of Kabir :
- The ways in which the major teachings of Kabir and Baba Guru Nanak were transmitted are as given below –
- Compositions of the sants were sung by roadside musicians.
- Baba Guru Nanak would sing his compositions in various ragas while his attendant Mardana played the rabab.
- Baba Guru Nanak organised his followers into a community. He set up rules for congregational worship (sangat) involving collective recitation.
Question 6.
Discuss the major beliefs and practices that characterised Sufism.
Solution :
After the advent of Islam in the early, middle ages , it saw a new movement in later part. The movement has had great impact and reach in the Indian subcontinent. It is called Sufi movement. The Sufi saints were mystics. Their preachings included:
1. Sufi saints did not subscribe to the theological and rigid interpretations of religious scriptures of Islam. They believed that the interpretation have to be based on individual experiences. This way the theological interpretations became flexible. Further the control of the orthodox religious leaders got weakened. This was a people centric move.
2. They rejected the high sounding rituals. They also emphasised on simplicity in religious traditions and rites.
3. Sufi saints prescribed devotion to Almighty as path to salvation. They even approved of singing and dancing as part of devotion. It is notable that classical Islam has forbidden singing, dancing and any music.
4. The most important theme of Sufi philosophy was that serving people is the true religion. With the objective of serving the poor people they also held Langar. Today also one can go to Ajmer and can partake in the Langar organised on the tomb of Nijammudin Auliya, the great Sufi saint.
5. Sufi saints also emphasised on the equality among people and oneness among all.
Question 7.
Examine how and why rulers tried to establish connections with the traditions of the Nayanars and the sufis.
Solution :
The rulers tried to establish connections with the traditions of the Nayanars and the sufis to claim divine support and proclaim their own power and status.
(a) Rulars and Sufis :
(i) The Turks who had set up Delhi Sultanate also required legitimation from the sufis because they resisted the insistence of the ulama on imposing sharva as state law because they anticipated opposition from their non-Muslim subjects. The sultans, therefore, sought the support of the Sufis who derived their authority directly from God and did not depend on jurists to interpret the sharva.
(ii) The sultans wanted to have the blessings of Sufi saints because it was believed that the Auliya could intercede with God in order to improve the material and spiritual conditions of ordinary human beings. The Sultans of Delhi set up charitable trusts as endowments for hospices and granted tax-free land.
(iii) Akbar maintained connections to get their blessings for new conquests, fulfilment of vows and birth of sons. He used to visit the Dargah of Muinuddin Chishti. He went there 14 times and gave generous gifts, which were recorded in imperial documents. For example, in 1568, he offered a huge cauldron (degh) to facilitate cooking for pilgrims. He also had a mosque constructed within the compound of the dargah.
(b) Rulers and Nayanars :
(i) The Chola kings tried to establish connections with Nayanars and Alvars by building splendid temples that were adorned with stone and metal sculpture to recreate the visions of these popular saints who sang in the language of the people.
(iii) Those kings also introduced the singing of Tamil Shaiva hymns in the temples under royal patronage taking initiative to collect and organise them into a text.
(iii) An inscription suggests that Chola ruler Parantaka I had consecrated metal images of Appar, Sambandar and Sundarar in a Shiva temple. These were carried in processions during the festivals of these saints.
Question 8.
Analyse, with illustrations, why bhakti and sufi thinkers adopted a variety of languages in which to express their opinions.
Solution :
The bhakti and sufi thinkers adopted a variety of languages to express their opinions due to the following reasons :
- The local languages were used so that people might understand their verses easily.
- The language of the Vedas was Sanskrit. The Bhakti traditions were against the beliefs and practices of the Vedas. So, it was necessary to use local languages which people might understand and follow them in practice. That is why Kabir’s poems have survived in several languages. Similarly, Baba Guru Nanak expressed his ideas through hymns called “shabad” in Punjabi, the language of the region.
- The Chishtis too adopted local languages such as Hindavi because poetic compositions were recited in hospices, usually during ‘sama’. In Bijapur, sufi poetry was composed in Dakhani because these were probably sung by women while performing household chores like grinding grain and spinning.
Question 9.
Read any five of the sources included in this chapter and discuss the social and religious ideas that are expressed in them.
Solution :
The period of the Bhakti Movement and Sufi Movement also has many sources that contribute to the history of those days. Some of the major social and religious ideas expressed in the various sources of history are as follows:
1. The first is the architecture. The different types of stupas, temple, monasteries all symbolise different types of religious belief system and practices. Some of them exist as it is and enable us to look into the annals of history of those days. Some of them are in the form of ruins but they also throw light on the, religion and society of those days alike.
2. The next important source of history is the composition of the saints both Bhakti
and Sufi. In terms of content they are religious but they are not the divine textbooks of religion that are sacrosanct. The compilation throws light on the life of common men and village lifestyle. They also impact the music and art of those days.
3. Another very important source of the history of those days is the biographies of the Saints. The biographies include the description of the society and prevalent beliefs and practices. It is notable that such biographies may not be in the written form still they can give insight into the life of those days. It is the story prevalent that I when Kabirdas died, both Hindus and Muslims fought for his dead body later on his body turned into flowers. Some were taken by Muslims and others by Hindus.
This represents that there conflict and collaboration between both Hindus and Muslims of those days.
4. This was also the period of rise of religious leaders who were intermediaries between common men and God. Earlier it was only the Brahmins who got this role. Now many people from other background also joined in. To some extent it acted as the the force that idolised equality and fraternity.
5. The other source is the folklore. They are described in our art forms. It may be dance, paintings, and sculpture and so on. They all talk about the universal brotherhood of mankind and love for one and all.
We hope the NCERT Solutions for Class 12 History Chapter 6 Bhakti-Sufi Traditions Changes in Religious Beliefs and Devotional Texts help you. If you have any query regarding NCERT Solutions for Class 12 History Chapter 6 Bhakti-Sufi Traditions Changes in Religious Beliefs and Devotional Texts, drop a comment below and we will get back to you at the earliest.