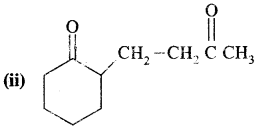
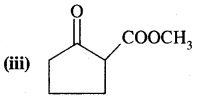
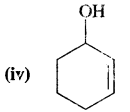
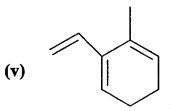
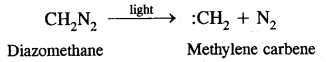
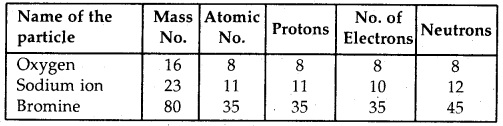
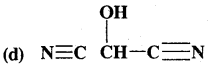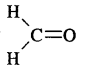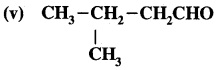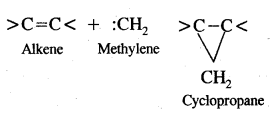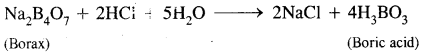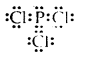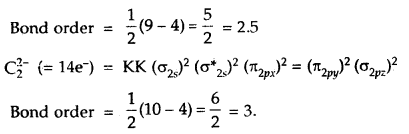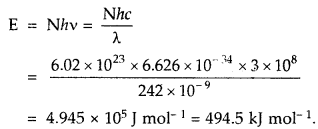Here we are providing Class 11 chemistry Important Extra Questions and Answers Chapter 14 Environmental Chemistry. Chemistry Class 11 Important Questions are the best resource for students which helps in Class 11 board exams.
Class 11 Chemistry Chapter 14 Important Extra Questions Environmental Chemistry
Environmental Chemistry Important Extra Questions Very Short Answer Type
Question 1.
What is the percentage of CO2 in pure dry air?
Answer:
About 0.03%.
Question 2.
Which gas was responsible for the Bhopal tragedy?
Answer:
Methyl isocyanate (MIC).
Question 3.
What is smog?
Answer:
A combination of smoke and fog.
Question 4.
Name three natural sources of air pollution.
Answer:
- Volcanic eruptions
- Forest fires
- Pollen grains of flowers.
Question 5.
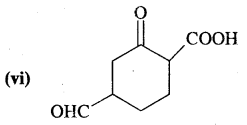
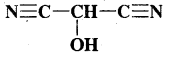
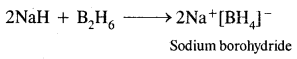
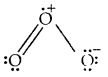
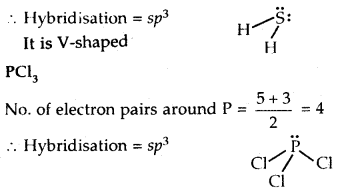
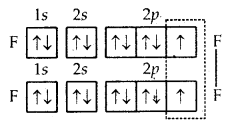
What is that compound formed when CO combines with blood?
Answer:
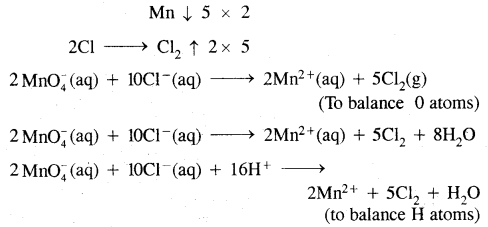
![]()
Question 6.
Name three gases that are major air pollutants.
Answer:
- CO2
- NO2
- SO2.
Question 7.
How does particulate help in cloud formation?
Answer:
The particulates work as nuclei for cloud formation.
Question 8.
Give one main reason for ozone depletion.
Answer:
The release of chlorofluorocarbons (CFCs) also known as freon.
Question 9.
Name two important sinks of CO.
Answer:
Oceans that dissolve it and plants use it for photosynthesis.
Question 10.
Which acids arc present in the added rain?
Answer:
- H2SO4
- HNO3
- HCl.
Question 11.
What is the nature of London smog?
Answer:
It is reducing in nature.
Question 12.
Name two herbicides used to kill weeds.
Answer:
- Sodium chlorate (NaClO3)
- Sodium arsenite (Na3AsO3)
Question 13.
Name two gases that form acid rain.
Answer:
- SO2
- NO2.
Question 14.
What is the composition of photochemical smog?
Answer:
It is a mixture of irritation causing compounds NO2, O3, hydrocarbons, acrolein, formaldehyde, peroxyacetyl nitrate (PAN).
Question 15.
Which zone is called the ozonosphere?
Answer:
Stratospheric zone.
Question 16.
Name any four methods of waste management.
Answer:
- Recycling
- Burning and incineration
- Sewage treatment
- Dumping.
Question 17.
What is BOD?
Answer:
The amount of oxygen consumed by microorganisms in decomposing waste in a sample of sewage water is called BOD (Biochemical Oxygen Demand).
Question 18.
What is the size range of particulates?
Answer:
5 nm to 500,000 nm.
Question 19.
What type of aromatic compounds are present as particulates in the air?
Answer:
Poly cyclic aromatic hydrocarbons [PAH].
Question 20.
In which season and what time of day, there is London smog?
Answer:
In winter during morning hours.
Question 21.
Which disease is caused due to a hole in the ozone layer and why?
Answer:
Ultraviolet rays from the sun will reach the earth after passing through the ozone hole and cause skin cancer.
Question 22.
What are polar stratospherical clouds (PSC)?
Answer:
The special type of clouds present over Antarctica in winter are called polar stratospheric clouds.
Question 23.
What should be the tolerable limit of fluoride ions in drinking water? What happens if it is more than 10 ppm?
Answer:
One ppm or 1 mg dm-3. The higher concentration is harmful to bones and teeth.
Question 24.
Which main compounds are causing damage to the ozone layer?
Answer:
NO and freons.
Question 25.
What is the composition of London smog?
Answer:
Fog of H2SO4 droplets deposited on the particulates.
Question 26.
Why photochemical smog is so called?
Answer:
Because it is formed as a result of the photochemical reaction between oxides of nitrogen and hydrocarbons.
Question 27.
In which season and what time of day, there is photochemical smog?
Answer:
In summer, in the afternoon.
Question 28.
What is the ‘nature of photochemical smog?
Answer:
It is oxidising in nature.
Question 29.
Name three methods used in green chemistry.
Answer:
Use of sunlight and microwaves, use of sound waves and use of enzymes.
Question 30.
Give three examples in which green chemistry has been applied.
Answer:
- Dry cleaning of clothes
- Bleaching of paper
- Synthesising chemicals.
Question 31.
What are the main advantages of using green chemistry?
Answer:
It is a cost-effective approach that involves a reduction in material, energy consumption and waste generation.
Question 32.
What is the troposphere?
Answer:
The troposphere is the lowest region of the atmosphere (~ 10 km) in which man along with other organisms including plants exist.
Question 33.
What is the stratosphere?
Answer:
The stratosphere extends above the troposphere up to 50 km above; sea level.
Question 34.
Name three scientists who got Noble Prize in chemistry in 2005 for their work in reducing hazardous waste in creating new chemicals.
Answer:
- Yves Chauvin
- Robert H. Grubbs
- Richard R. Schrock.
Question 35.
What is meant by metathesis?
Answer:
Metathesis is an example of how important basic science has been applied for the benefit of man, society and the environment.
Question 36.
In what regions of the atmosphere, the temperature increases with altitude and in which regions it decreases?
Answer:
Temperature increases with altitude in the stratosphere and thermosphere and it decreases with altitude in the troposphere and mesosphere.
Question 37.
What do you mean by ‘Inversion temperature’ in different regions of the atmosphere?
Answer:
When we go from one region of the atmosphere to the next adjoining region, the trend of temperature changes from increase to decrease or vice-versa is called inversion temperature.
Question 38.
What is the most important sink of CO pollutant?
Answer:
Soil micro-organisms.
Question 39.
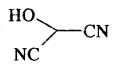
What is the compound formed when CO combines with blood?
Answer:
Carboxyhaemoglobin (HbCO).
Question 40.
What is anoxia starvation in the body (due to CO poisoning)?
Answer:
Actual oxygen starvation in the body (due to CO poisoning).
Question 41.
How are the flue gases from industries freed from oxides of N and S?
Answer:
By scrubbing them with a cone. H2SO4 or with alkaline solutions of Ca(OH)2 & Mg(OH)2.
Question 42.
What is chlorosis?
Answer:
Slowing down the formation of chlorophyll in plants due to the presence of SO2 as a pollutant is called chlorosis.
Question 43.
What is the cause of blue baby syndrome?
Answer:
Excess of nitrates in drinking water leads to methemoglobinemia which is commonly called ‘blue baby syndrome.
Question 44.
Give I.U.P.A.C. name of B.H.C.
Answer:
1, 2, 3, 4, 5, 6 Hexa- chloro cyclohexane.
Question 45.
What is meant by P.C.Bs.?
Answer:
P.C.Bs. are polychlorinated biphenyls. They are contaminants of water.
Question 46.
What is P.A.N.?
Answer:
Peroxy acetyl nitrate.
Question 47.
Name the oxidizing agent used in determining C.O.D.
Answer:
Potassium dichromate (K2C2O7).
Question 48.
What are fungicides?
Answer:
The chemicals which check the growth of fungi are called fungicides.
Question 49.
When does rainwater become Acid rain?
Answer:
When the pH of rainwater becomes as low as 2 to 3.5, it forms acid rain.
Question 50.
What is meant by Taj Trapezium?
Answer:
Taj Trapezium is the area that includes the town of Agra, Firozabad, Mathura & Bharatpur. Under this plan, more than 2000 polluting industries lying inside the Trapezium would switch over to the use of natural gas or L.P.G. instead of coal or oil.
Environmental Chemistry Important Extra Questions Short Answer Type
Question 1.
Fish do not grow as well in warm water as in cold water. Why?
Answer:
The amount of dissolved oxygen in warm water is less than in cold water. For the fish to survive in water, the concentration of dissolved O2 is 6 ppm which decreases in warm water.
Question 2.
Why does rainwater normally have a pH of about 5.6? When does it become acid rain?
Answer:
Normally rain has a pH of about 5.6 due to the dissolution of CO2 of the atmosphere into it.
CO2(g) + H20O(l) → H2CO3(al)
H2CO3(aq) ⇌ 2H+ + CO32-
When the pH of rain falls below 5.6, it becomes acid rain.
Question 3.
In which season the depletion of ozone in Antarctica takes place and when is it replenished?
Answer:
During spring seasons (i.e., in the months of September and October) depletion of ozone takes place and after spring (in the month of November) it is replenished.
Question 4.
What is the role of CO2 in the greenhouse effect?
Answer:
The heat from the sun after being absorbed by the earth is remitted by the earth and absorbed by CO2 and then radiated back to the earth, thereby warming it.
Question 5.
What are viable and non-viable particulates?
Answer:
Viable particulates are small-sized living organisms such as bacteria, fungi, moulds and algae etc. Non-viable particulates are formed by the disintegration of large-sized materials or condensation of small-sized particles or droplets e.g. mist, smoke, fume and dust.
Question 6.
Why there is ozone depletion mainly over Antarctica?
Answer:
This is because, in other parts of the stratosphere, chlorine-free radicals combine away but in Antarctica, the compounds formed are converted back to chlorine-free radicals which deplete the ozone layer.
Question 7.
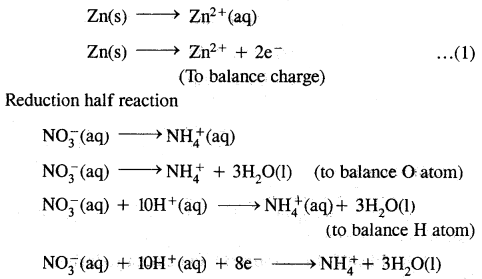
Explain giving reasons. “The presence of CO reduces the amount of haemoglobin available in the blood for carrying oxygen to the body cells”.
Answer:
CO combines with haemoglobin of the red blood corpuscles (R.B.Cs) about 300 times more easily than oxygen to form carboxyhaemoglobin reversibly as follows:
Hb + CO ⇌ HbCO
Thus it is not able to combine with oxygen to form oxyhaemoglobin and transport of oxygen to different body cells cannot take place.
Question 8.
Why is acid rain considered a threat to the Taj Mahal?
Answer:
Taj Mahal is made of white marble (CaCO3). The acid rain contains H2SO4 in a very dilute form which attacks marble, thereby pitting it, discolouring it and making it lustreless.
CaCO3(s) + H2SO4 (aq) → CaSO4(aq) + CO2(g) + H2O(l)
Question 9.
What are the harmful effects of carbon monoxide?
Answer:
1. Carbon mono-oxide when inhaled passed through the lungs into the blood where it reacts with the haemoglobin of the die R.B.C. to form a complex. Known as Carboxy haemoglobin the latter is not in a position to transport the inhaled oxygen to various parts of the body. This will cause suffocation & will ultimately lead to death.
2. A high concentration of carbon mono-oxide (100 ppm) or more will harmfully affect the plants causing leaf drop reduction in leaf size & pre-mature ageing etc.
Question 10.
What are the harmful effects of oxides of nitrogen?
Answer:
- A high concentration of NO2 in the atmosphere is harmful to plants resulting in leaf spotting, retardation of photosynthetic activity & also suppression the vegetation growth.
- NO2 results in respiratory problems in human beings & lead to bronchitis.
- NO2 has harmful effects on nylon, rayon & cotton yarns & also cause cracks in the rubber.
- They also react with ozone present in the atmosphere & thus decrease the density of ozone.
Question 11.
What are the harmful effects of oxides of Sulphur?
Answer:
- SO2 is a major source of irritation for the eyes & respiratory tract & leads to serious diseases such as bronchitis & lung cancer even when it is about 2.5 ppm. in the atmosphere.
- London smog & sulphurous acid smog which mainly consists of SO2(0-40 ppm or above) is known as smog killer.
- Plants are relatively more sensitive to the harmful effect of SO2 as compared to animals. Constant exposure to air containing a high level of SO2 kills leaf tissues, reduces plants productivity & also bleaches leaf pigments.
- SO2 has harmful effects on buildings & statues made up of marble & limestone.
- Air polluted with oxides of sulphur also accelerates the corrosion of metals like copper, zinc, iron etc.
Question 12.
How do detergents cause water pollution?
Answer:
- Detergents are non-biodegradable & they cause water pollution.
- They inhibit the oxidation of organic substances present in wastewaters because they form a sort of Envelope around them.
- They form stable foam in rivers that extend over several hundred meters of the river water.
Question 13.
Why is add rain considered a threat to the Taj Mahal?
Answer:
The air of the city Agra, where the Taj Mahal is located, contains a very high level of Sulphur & nitrogen oxides. The resulting acid rain reacts with the marble of the Taj Mahal. Causing pitting in this wonderful monument that has attracted people from around the world. As a result, the monument is being slowly eaten away & the marble is getting decoloured & lusterless.
Question 14.
Explain giving reasons “The presence of co-reduces the amount of haemoglobin available in the blood for carrying oxygen to the body cells?
Answer:
Carbon mono-oxide binds itself with the haemoglobin of the R.B.Cs. about 200 times more easily than oxygen to form carboxyhaemoglobin reversibly as follows:
Hb + CO ⇌ HbCO
The presence of CO, therefore, reduces the amount of haemoglobin available in the blood for the transport of oxygen to the body cells & therefore, with fewer O2 levels, normal metabolism is impaired.
Question 15.
What do you understand by the greenhouse effect? What are the major greenhouse gases?
Answer:
The greenhouse effect is the phenomenon in which the earth’s atmosphere traps the heat from the sun, & prevents it from escaping into outer space. The warming of the earth or global warming due to re-emission of sun’s energy absorbed by the earth followed by its adsorption by CO2 molecules & H2O vapours present near the earth’s surface & then it’s radiation back to the earth is called “Greenhouse effect”. The major greenhouse gases are:
CO2, CH4, C.F.Cs & water vapours.
Question 16.
What do you understand by Mists, Smoke, Fumes & Dust?
Answer:
Mists: Mists are produced by particles of spray liquids & the condensation of vapours in the air. Examples are portions of herbicides & insecticides, that miss their targets & travel through the air to form mists.
Smoke: They are very small soot particles produced by burning & combustion of organic matter. Oil smoke, Tobacco smoke & Carbon smoke are typical examples.
Dust: It consists of fine particle produced during crushing, grinding & attribution of solid materials. Non-viable dust particulates in the atmosphere consist of ground limestone, sand tailing from floatation, pulverised coal, cement, fly ash & silica dust.
Question 17.
What is Pneumo coniosis? How does it occur?
Answer:
The smaller particulate pollutants are more likely to penetrate into the lungs. These fine particles are carcinogens. Inhalation of small particles irritates the lungs & exposure to such particles for long periods of time causes fibrosis of lung lining. This type of disease is termed “Pneumoconiosis”. This occurs due to exposure to such particles for a long time.
Question 18.
What measures should be taken to check pollution by sewage?
Answer:
- Sewage must be churned by machines so that the large pieces may break into smaller ones & may get mixed thoroughly. The churned sewage is passed into a tank with a gentle slope. Heavier particles settle & the water flowing down is relatively pure.
- Water must be sterilized with the help of chlorination. Chlorination is very essential, particularly in rainy session.
- Treatment of water with alum, lime etc.
Question 19.
Discuss the water pollution caused by industrial wastes.
Answer:
The compounds of lead, mercury, cadmium, nickel, cobalt & zinc etc. Which are the products of chemical reactions; carried in the industrial units pollute water to a large extent & are responsible for many diseases. Mercury leads to Minamata disease, & lead poisoning leads to various types of deformities.
Question 20.
What remedial steps should be taken to save a person suffering from co-poisoning?
Answer:
Remedial treatment for CO poisoning:
- Carry the patient into the fresh air immediately & do not allow him to walk.
- Lose his clothes & take off his shoes.
- Give artificial respiration if the patient is not able to breathe properly.
- In the hospital the patient should be kept in a high-pressure chamber containing oxygen at 2-2.5 atm pressure under pressure, CO of carboxyhaemoglobin is replaced by O2 & thus the transport of O2 to different parts of the body starts.
HbCO + O2 ⇌ HbO2 + CO
Question 21.
How can pollution due to nitrogen & Sulphur oxides be controlled?
Answer:
The following steps are taken to control NO2 & SO2 pollution:
(i) The catalytic converters should be used in the automobile exhausts which is the first stage of converting the oxides of nitrogen, to free N2 or to a small amount of NH3.
(ii) The fumes of gases coming from power plants or industrial, units & containing NO2 & SO2 are freed from these gases by scrubbing the fumes with H2SO4. The following reactions take place:
- I- step: NO2 + SO2 + H2O → H2SO4 + NO
- II- step: NO + NO2 → N2O3
- III- step: N2O3 + 2H2SO4 → 2NOHSO4 + H2O
the fumes of gases are thus freed from NO2 & SO2 & are released into the atmosphere. The reaction product NOH2O4 is decomposed to get H2SO4 which is then used again for scrubbing. As NOx & SOx are > acidic oxides, scrubbing can also be done with an alkaline solution such as Ca(OH)2 & Mg(OH)2.
Question 22.
What is groundwater pollution? How does it take place?
Answer:
Water below the surface of the earth is called groundwater. Most of the freshwater (> 90%) is present as groundwater. The remaining is present as groundwater. The remaining is present in lakes, ponds, rivers, streams etc. only 2% of water is present as soil moisture above the water table, which is needed for the growth of the plants.
Groundwater collected below the surface of the earth after passing through the pores of earthy materials which acts as a filter for it & is pure. It is for this reason that well water is spring water is used for drinking purpose in rural areas. However, due to the disposal of domestic wastes, industrial effluents, use of fertilizers & pesticides in agriculture, A number of harmful soluble substance dissolve into the rainwater & result in groundwater pollution especially where the water table is high.
Question 23.
How is the pollution of river water caused in India? What measures have been taken by the Government to check river pollution?
Answer:
The main reasons for pollution in rivers are as follows:
- Industrial waste discharge: It includes those from paper, textiles, fertilizers, rayon, pesticides, detergents, drug industries & refineries.
- Domestic sewage discharge: The government has enacted laws banning the discharge of industrial or domestic wastes into these rivers. It has started the following plans to clean up the water of these rivers.
(a) Ganga Action Plan I & II
(b) Yamuna Action Plan
(c) Plan to clean Hoogly water.
Question 24.
What are the effects of oil pollution on Sea Water?
Answer:
Effects of oil pollution on Sea Water:
- oil spills cause heavy damage to fishes oil coating makes them unable to respire & clogs their gill slits. Aromatic compounds present in them are poison for fishes.
- Emulsified oil goes deep down into the sea damaging aquatic animals & plants.
- Oil spills result in a reduction of dissolved oxygen.
- The most affected by oil pollution are the sea birds. Natural, insulating oil & waxes which shield the birds from water are broken down by the spilt oil. As a result due to loss of insulation, they start shivering & are freezing to death, especially in winter.
Question 25.
Which is the permitted safety limit of fluoride & lead concentration with respect to international standards of drinking water?
Answer:
The fluoride concentration should be about 1 ppm in drinking water. This concentration is within agreed safety limits & has been shown to protect teeth against decay. A high concentration of fluoride is poisonous & are harmful to bones & teeth at levels over 10 ppm.
The lead concentration should be about 50 ppm in drinking water.
Question 26.
Define the term pesticides? What are three categories of pesticides?
Answer:
Pesticides are substances that are used to control the reproductive process of unwanted organisms.
Three main categories of pesticides are:
- Insecticides: These are used to control insects & curb diseases (Malaria, Yellow fever) & protect crops e.g. D.D.T.
- Herbicides: These are used to kill weeds eg. NaClO3 & Na2AsO3
- Fungicides: These are used to check the growth of fungi e.g. Methyl mercury.
Question 27.
Write four major pollutants of water, their source & effects.
Answer:
| S.No. Major Pollutants of Water | Sources | Effects |
| 1. Lead | Lead-acid batteries & leaded gasoline | Toxic to organisms causes phimosis |
| 2. Acids | Mine drainages, Industrial wastes | Kills organisms, overgrowth of algae & aquatic weeds. |
| 3. Detergents | Households & industries | Depletion of dissolved oxygen. |
| 4. Pesticides & Insecticides | Agriculture & Mosquitoes repellants | Toxic to fishes, birds & mammals. |
Question 28.
Discuss the mechanism of treatment of industrial wastes.
Answer:
The treatment of industrial waste depends upon the nature of the pollutant present. In order to ascertain it, the pH of a medium is first determined & the waste is then neutralized with the help of suitable acids or alkalies.
The chemical substances present in the industrial waste product, dissolved in water can be precipitated by suitable chemical reactions & removed later on from water. Quite recently, photo-catalysis & ion exchangers have been developed for the treatment of industrial wastes.
Question 29.
What measures are necessary to control soil pollution?
Answer:
In order to control soil pollution, the following measures are necessary:
- Use of manures: Manures is semi-decayed organic matter which is added to the soil to maintain its fertility. These are mostly prepared from animal dung & another form of wastes. These are much better than the commonly used fertilizers.
- Use of bio-fertilizers: These are organisms that are inoculated in order to bring about nutrient enrichment of the soil e.g. nitrogen-fixing bacteria & blue-green algae.
- Proper sewerage System; A proper sewerage system must be employed & sewerage recycling plants must be installed in all town & cities.
- Salvage & Recycling: Rag-picking removes a large no. of waste articles such s paper, polythene, cardboard rags, empty bottles & metallic, articles. These are subjected to recycling & this helps in checking soil pollution.
Question 30.
What are aerosols? Define the term green chemistry.
Answer:
Very small particles of solids & liquids dispersed in the air are called aerosols. Aerosol particles have a diameter of less than one micrometre (< 1μm). The particles of aerosols are so small that they remain suspended in the air.
Green Chemistry: The branch of chemistry which emphasizes the process & products that reduce or eliminate the use & generation of toxic/hazardous substances is called Green Chemistry.
Environmental Chemistry Important Extra Questions Long Answer Type
Question 1.
What is the difference between London (classical) smog and photochemical (Los Angeles) smog?
Answer:
| Classical (London) smog | Photochemical (Los Angeles) Smog |
| 1. This type of smog was first observed in London in 1952. | 1. This type of smog was first observed in Los Angeles in 1950. |
| 2. It is formed due to the presence of SO2 and humidity in the air which combines to form H2S04 fog which gets deposited on the particulates. | 2. It is formed due to a photochemical reaction taking place when the air contains NO2 and hydrocarbons. |
| 3. It involves smoke and fog. | 3. It does not involve any smoke or fog. The word smog is a misnomer here. |
| 4. It is formed in the months of winter particularly in the morning hours when the temperature is low. | 4. It is formed during the months of summer during the afternoon when there is bright sunlight so that photochemical reaction can take place. |
| 5. It causes a problem in the lungs. | 5. It causes irritation in the eyes. |
| 6. It is reducing in character. | 6. It is oxidising in character. |
Question 2.
(a) Mention some of the sources of soil pollution.
Answer:
Some of the sources of soil pollution are:
- Industrial wastes.
- Urban wastes
- Agriculture pollutants include synthetic fertilizers, pesticides, insecticides like DDT, BHC, herbicides like NaClO3, Na3AsO3, fungicides soil conditioners, farm wastes.
- Radioactive pollutants.
(b) Mention some ways to control environmental pollution.
Answer:
Environmental pollutants like household waste and industrial waste can be controlled in the following manner.
- Recycling: Used glass bottles, iron scrap, plastic waste, polythene bags, used newspapers and magazines can all be properly recycled.
- Burning and incineration.
- Sewage treatment
- Digesting.
- Dumping
- Green chemistry can be used to reduce pollution.