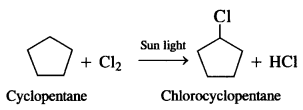
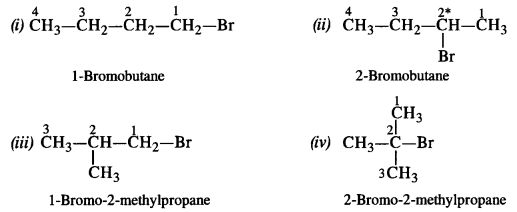
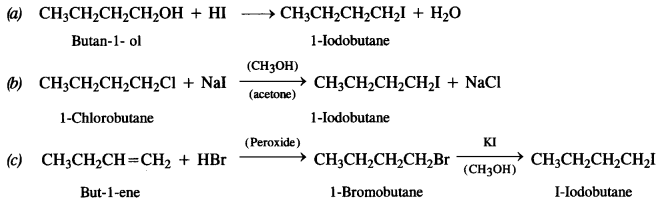
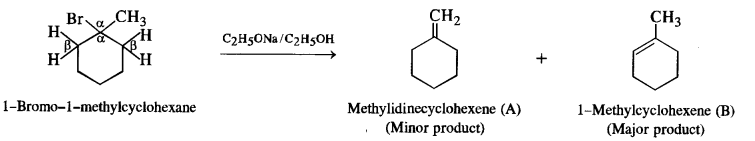
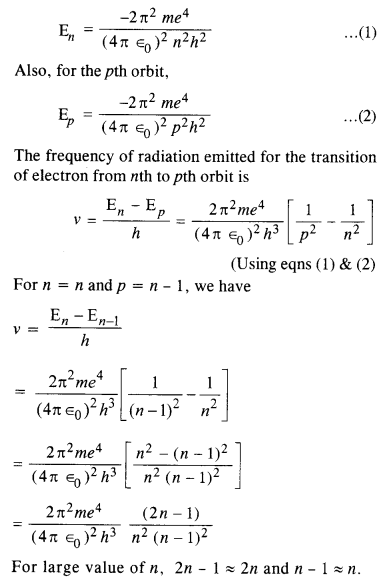
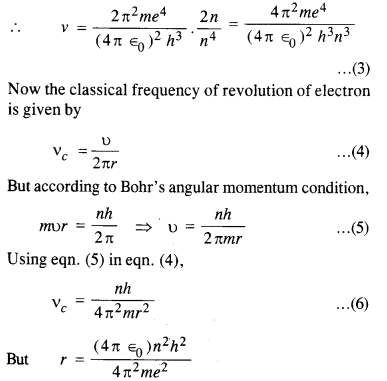
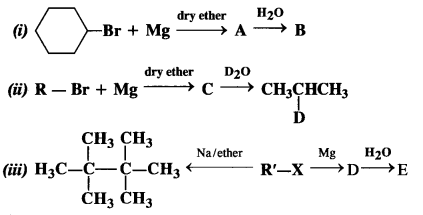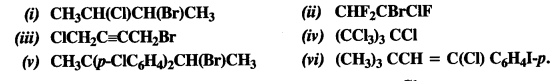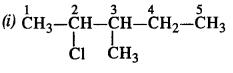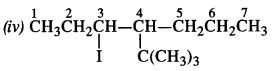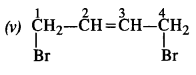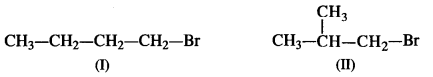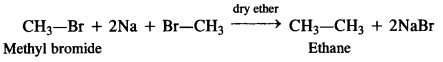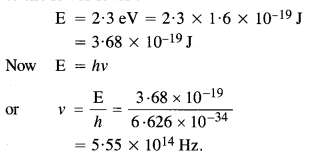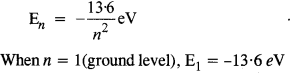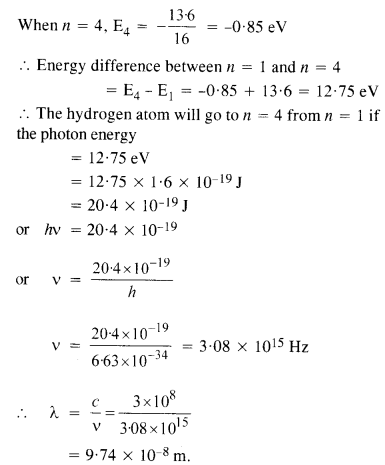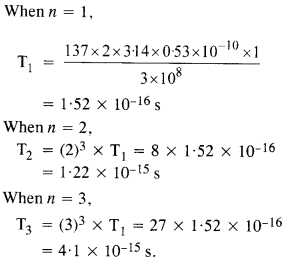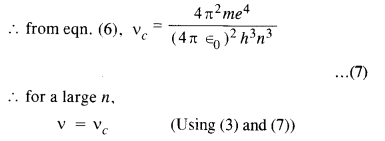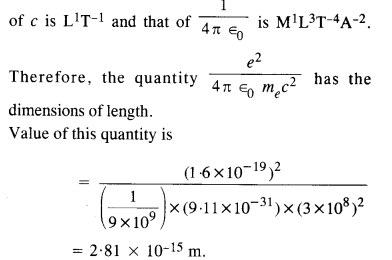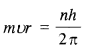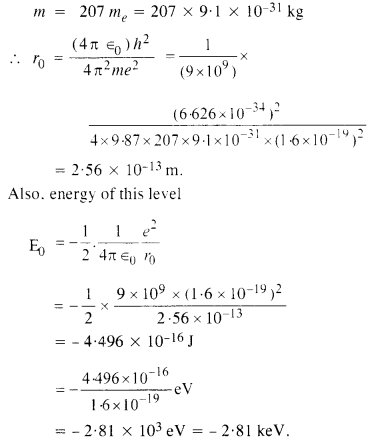Here we are providing NCERT Solutions for Class 12 English Flamingo Poem 6 Aunt Jennifer’s Tigers. Students can get Class 12 English Aunt Jennifer’s Tigers NCERT Solutions, Questions and Answers designed by subject expert teachers.
Aunt Jennifer’s Tigers NCERT Solutions for Class 12 English Flamingo Poem 6
Aunt Jennifer’s Tigers NCERT Text Book Questions and Answers
Aunt Jennifer’s Tigers Think it out
Question 1.
How do “denizens” and “chivalric” add to our understanding of the tiger’s attitudes?
Answer:
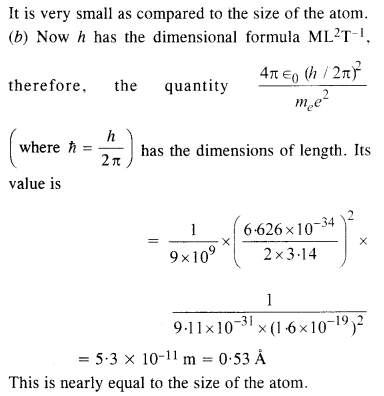
Unlike their creator who sunk into the background, the tigers were described as “bright topaz denizens”. The tigers were energetic and free to “prance” or run around in the jungle, and in their creator’s imagination. The tigers were created bright, like “topaz”, and they inhabited a world that is green. The “bright topaz denizens of green” evoke a mental image of majestic tigers not bound by the whims of another being. They are in their natural environment. The tigers are conceived as inherently male, they are chivalric, hence tied to the long tradition of male authority and power. However, their “chivalric certainty” is a representation of the power envisioned by Aunt Jennifer for herself. This idea is then contrasted with Aunt Jennifer’s reality where she in turn was dominated by male superiority.
Question 2.
Why do you think Aunt Jennifer’s hands are “fluttering” through her wool in the second stanza? Why is she finding the needle so hard to pull?
Answer:
Aunt Jennifer worked with a piece of wool, stitching patterns into a tapestry. Her fingers fluttered to create the beautiful image of the tigers. She expressed her desires by creating the tigers. She found it difficult to express her feelings, repressed by the weight of marriage, gender roles, and a dominating society. “Uncle’s wedding band” is representative of the patriarchal society she lived in. The weight of the ring was not something she enjoyed as the band is described to sit ‘heavily’ on her hand and kept her from the only mode of expression she had, her needlepoint.
Question 3.
What is suggested by the image “massive weight of Uncle’s wedding band”?
Answer:
The struggle for existence in a harsh world, the deep conflicts of bondage and freedom and gender conflict is portrayed through the use of the evocative image. The genders are polarised. Aunt Jennifer was victimized by the absent Uncle, represented only by his wedding band, while he is representative of the oppression of custom and law.
Question 4.
Why is Aunt Jennifer terrified in the third stanza?
Answer:
In the last stanza, the reader is told that “the tigers in the panel she made/Will go on prancing, proud and unafraid” even after Aunt Jennifer was dead. This showed her fear. The tigers represent her spirit and how she would like to live while her hands, folded even in death, represent the reality of her life.
Question 5.
What are the ordeals Aunt Jennifer is surrounded by? Why is it significant that the poet uses the word “ringed”? What are the meanings of the word “ringed” in the poem?
Answer:
The word “ringed” has a double association. It indicates not only the ring that “sits heavily” on her hand, but the difficulties in her life that would continue to surround her.
Even in death, she is seen to conform to the patriarchal society in which she lived. The ring on her finger symbolised the weight she had to bear, dead or alive. Just as she created and controlled her needlework, society and gender roles created and controlled her.
Question 6.
Why do you think Aunt Jennifer created animals that are so different from her own character? What might the poet be suggesting, through this difference?
Answer:
Aunt Jennifer created an alternative world of freedom, one which she could not inhabit otherwise. The tigers of her creation represented her suppressed desires and her ambition. She was victimised, and stifled in marriage, by the absent figure of the Uncle, represented only by his wedding band. She escaped her reality through her creation. The poet presents an ironic image of a contrast between the common perception of animals as being brutal and of men being humane. Here, the ‘brutal’ tiger represents freedom while the ‘civilised’ man is exposed as the oppressor.
Question 7.
Interpret the symbols found in this poem.
Answer:
Adrienne Rich uses a number of similes and symbols in the poem to convey her theme. The tigers, of course, symbolise the freedom of spirit which Aunt Jennifer dreams of attaining but never achieves except in her dreams and her art. Aunt Jennifer represents of her gender rather than any one individual.
The tigers are symbolic of the true nature of the freedom that a woman’s soul represents. They also display in art the values that Aunt Jennifer must repress or displace in life: strength, assertion, fearlessness and fluidity of motion. The image of the tiger is both inspiring and destructive. And the poem’s conclusion celebrates the animal images as a kind of triumph, transcending the constraints of their maker’s life.
The word ‘ringed’ has a double connotation—indicating not only the ring that “sits heavily” on her hand, but the difficulties in her life that will continue to surround her.
Question 8.
Do you sympathise with Aunt Jennifer? What is the attitude of the poet towards Aunt Jennifer?
Answer:
As a reader, one sympathises with Aunt Jennifer. She aspired for a greater future and a greater standing for her generation. Her mind was liberated from the cloistered association with her sex, but the figure of Aunt Jennifer never got to see women standing strong and proud. In the end, Adrienne Rich showed that Aunt Jennifer represented every woman of her time. Ironically enough, she rebels using the oppressor’s own language to feel a sense of triumph. Overwhelmed by gender roles, and tom between rebellion, the need for expression, and society, Aunt Jennifer expressed her fears and desires through the exotic images of tigers, transcending her dreams and ambitions.
Aunt Jennifer’s Tigers Extra Questions and Answers
Aunt Jennifer’s Tigers Short Answer Questions
Question 1.
What are the characteristics of the tigers?
Answer:
The tigers have a striking appearance as they are described as “bright topaz”. They are energetic and prance about. They enjoy their freedom and hence run across the screen. The majestic tigers are not dictated by the whims of others. They are fearless and chivalrous. The elegant tigers are confident of themselves.
Question 2.
What do you learn about Aunt Jennifer?
Answer:
Aunt Jennifer was a weak woman with fluttering fingers. She was terrified of Uncle and found it difficult to manoeuvre the needle. Thus, implying that she was oppressed by a burdensome marriage where she was subjugated by Uncle.
Question 3.
What does Aunt Jennifer’s creation of the tigers symbolize?
Answer:
Aunt Jennifer’s creation of the tigers symbolizes her desires. Her fingers flutter to create the beautiful image of the tigers. By creating those tigers, she lets loose her inner aspirations. The wedding band sits “heavily” on her hand and keeps her from the only sense of expression she has—her needlepoint.
Question 4.
What does Aunt Jennifer’s death signify?
Answer:
In the last stanza of the poem, the poet gives us a surprisingly truthful look at the reality and the end of Aunt Jennifer and women in her position all over the world. Aunt Jennifer is a subjugated even in death; she must conform to the norms of the patriarchal society. The ring around her finger symbolizes the weight she must bear dead or alive.
Question 6.
What is the theme of the poem?
Answer:
“Aunt Jennifer’s Tigers” clearly reflects the gender struggle that women across the world are subject to. It is a feminist poem in which the poet criticizes the male-dominated world. Aunt Jennifer is left no option than to create an alternate world of freedom. Aunt Jennifer is a woman trapped by the social and cultural expectations and demands of her time.
Question 7.
Who do you think is the speaker of the poem? Why do you say so?
Answer:
The speaker of the poem is the niece; the word “Aunt” shows her relationship with the speaker. The point of view here would seem to be that of a woman, indicating that the speaker is the niece of Aunt Jennifer’s. The niece voices a woman’s struggles with expression, rebellion, and a society where power is defined as masculine. The poem deals with a woman’s representation of Aunt Jennifer’s dreams, reality and the future.
Question 8.
Interpret the following symbols found in this poem.
(a) tigers
(b) Aunt
(c) embroidery
(a) The tigers symbolize the freedom of spirit which Jennifer dreams of attaining but never achieves except in her dreams and her art.
(b) Aunt Jennifer is symbolic of women as a whole rather than one individual.
(c) Aunt Jennifer’s embroidery may exist forever as the work that she leaves behind; in life, she was nothing like the tigers in her embroidery.
Aunt Jennifer’s Tigers Value Based Question
Question 1.
How does the poet advocate gender sensitisation in the poem?
Answer:
The poem reflects the gender struggle; Adrienne Rich criticizes the male-dominated world for terrifying and oppressing women as symbolized by Aunt Jennifer, leaving her with no alternative but to create an alternate world of freedom for herself with her sewing. The embroidering of tigers on the panel, her only form of expression, underlines a woman’s struggles with expression, rebellion, and a society where power is defined as masculine. The poet depicts the pain of a woman who is living with a husband who dominates her. Her hidden, vibrant inner life is in sharp contrast to the outer image of the terrified, trapped woman.
The poem is almost a tragedy relating the plight of women trapped in an unhappy marriage. The poet makes her stance clear by using the figure of independent and fearless tigers as a telling symbol of an ideal that women like Aunt Jennifer seek to approximate. Adrienne Rich yearns for freedom and equality for all women.
Give examples from the poem of the following poetic devices.
Alliteration
“fingers fluttering”
“chivalric certainty”
Symbols
Aunt Jennifer
Tigers
Embroidery
wedding band
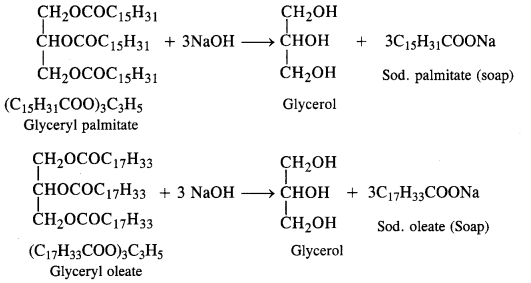
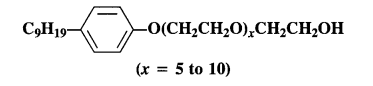
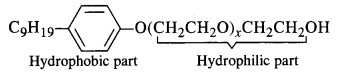
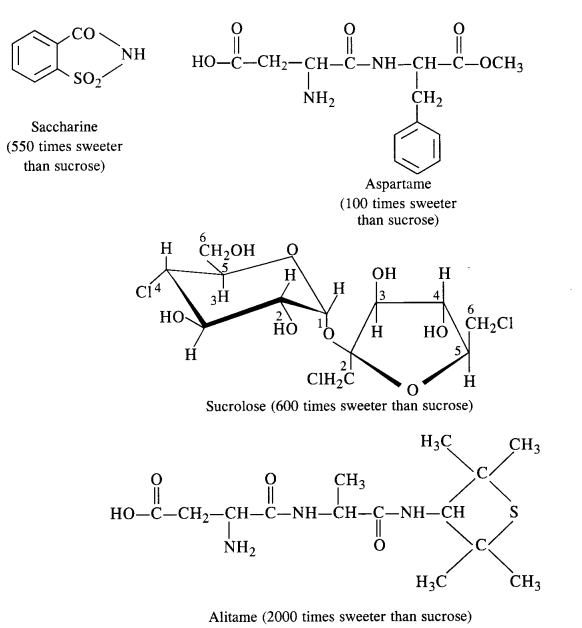
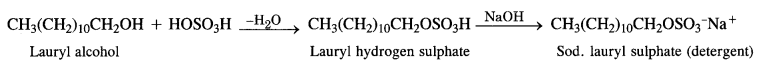
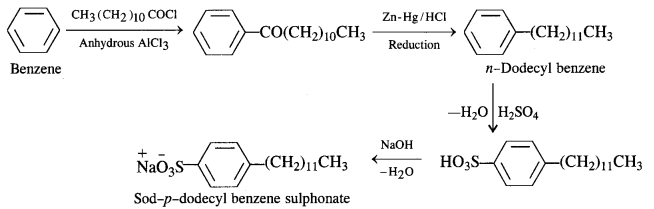
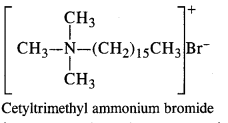
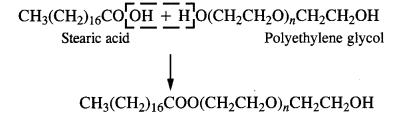
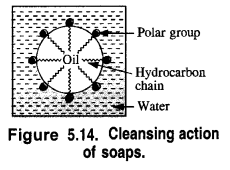
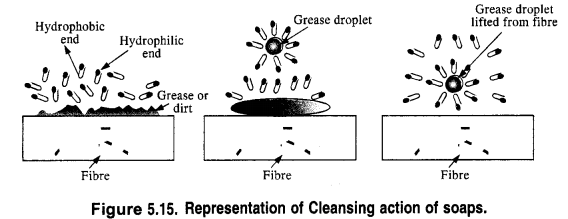
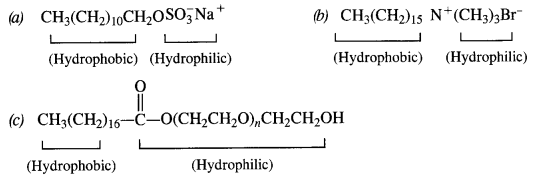

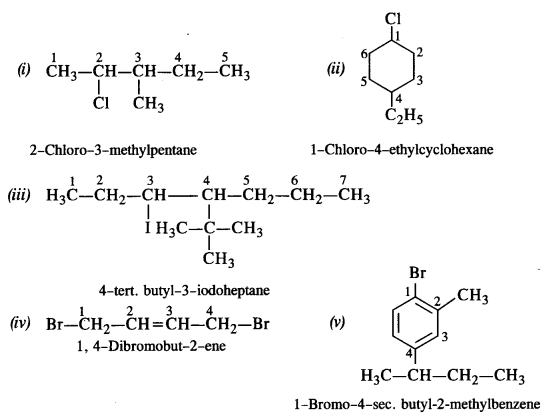
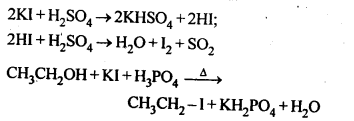
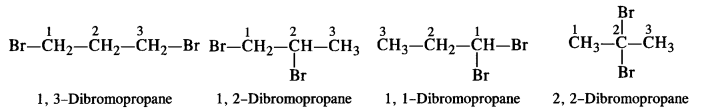
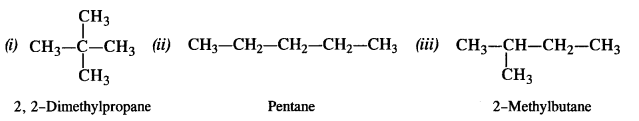
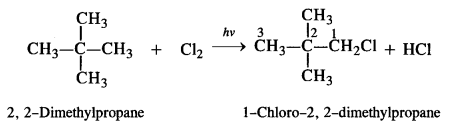
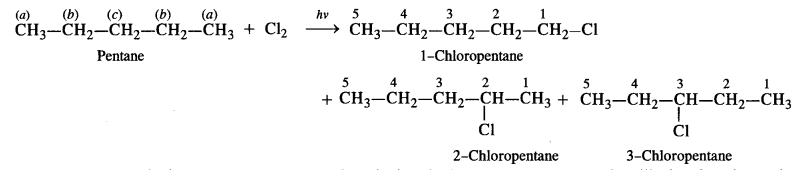
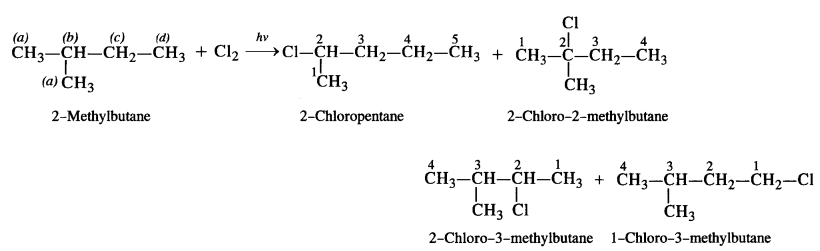
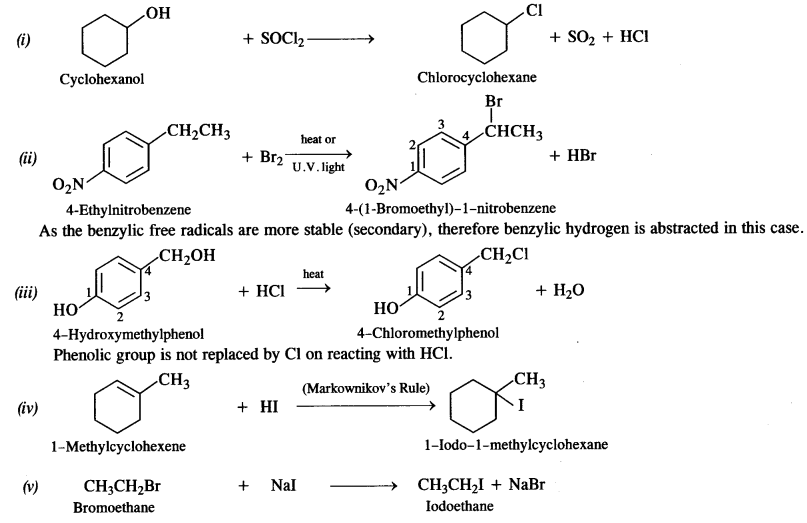
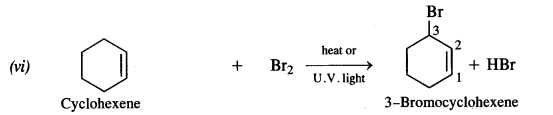
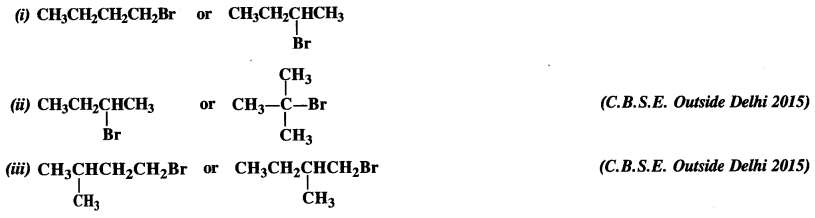
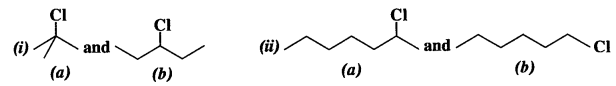
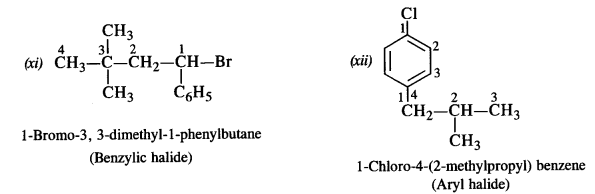
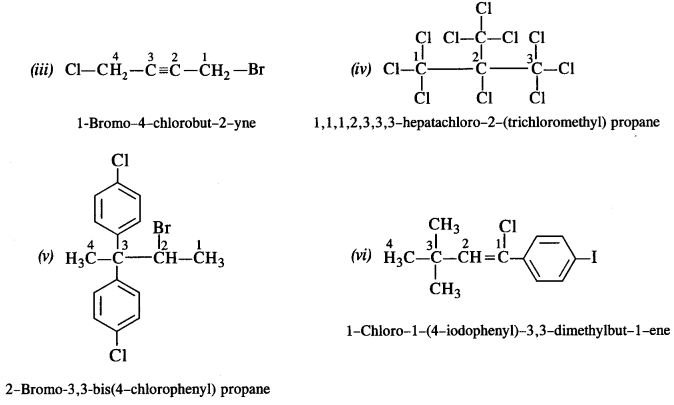
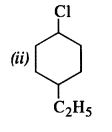
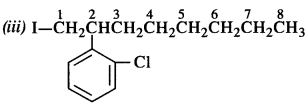
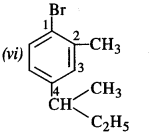
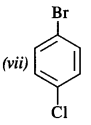
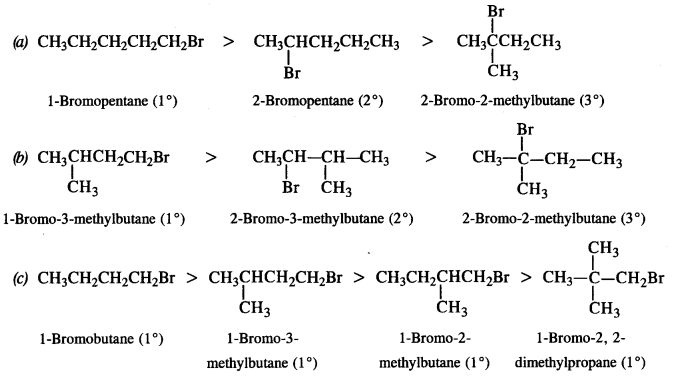
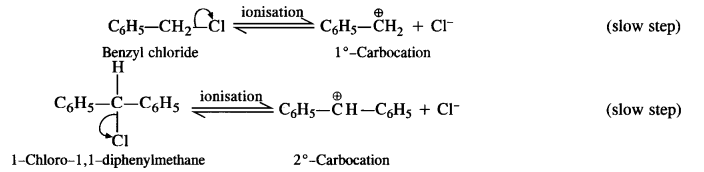
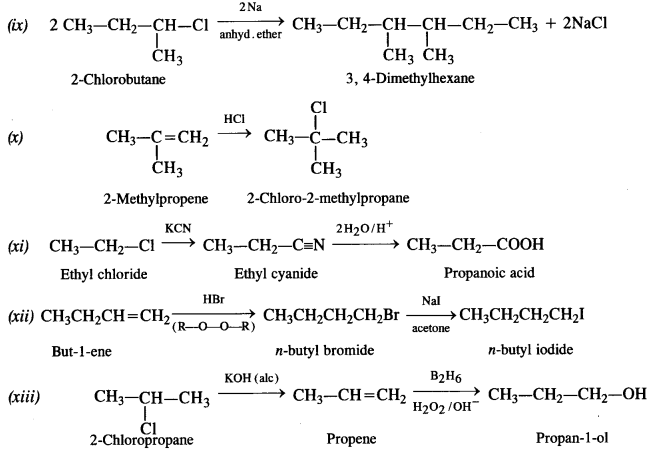
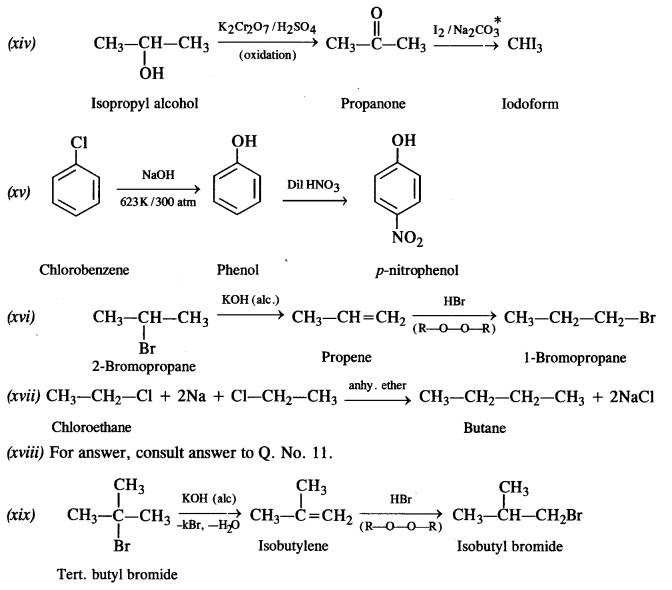
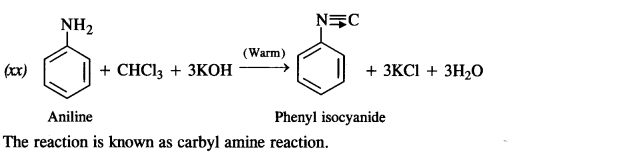
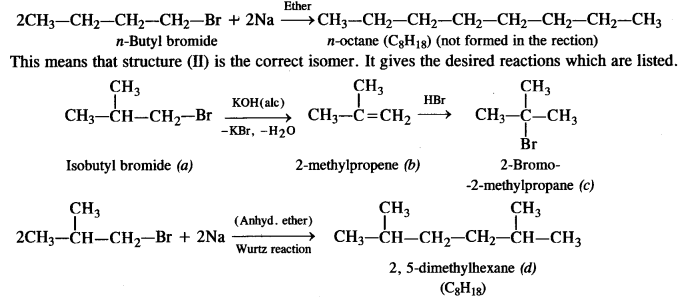
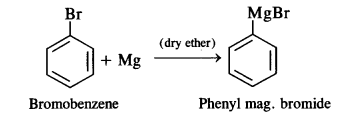
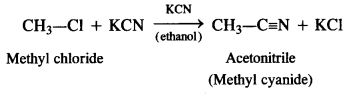
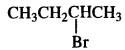 is a secondary alkyl halide (2°). It is more reactive than the other isomer which is a tertiary alkyl halide (3°). The explanation is the same.
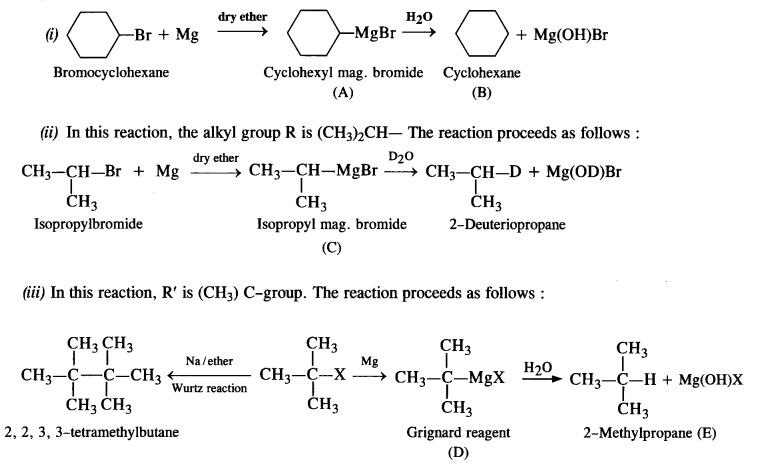
is a secondary alkyl halide (2°). It is more reactive than the other isomer which is a tertiary alkyl halide (3°). The explanation is the same.