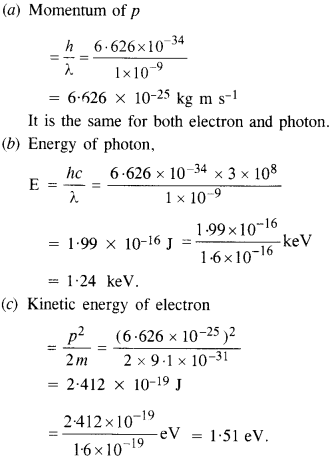
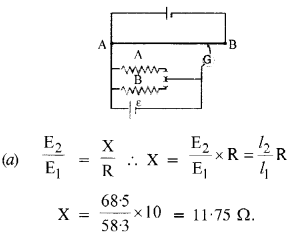
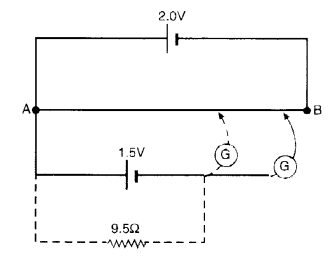
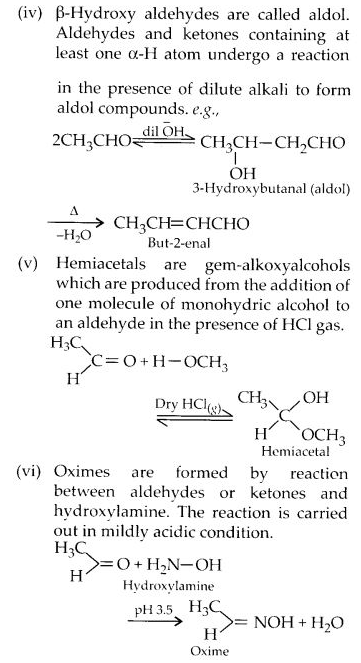
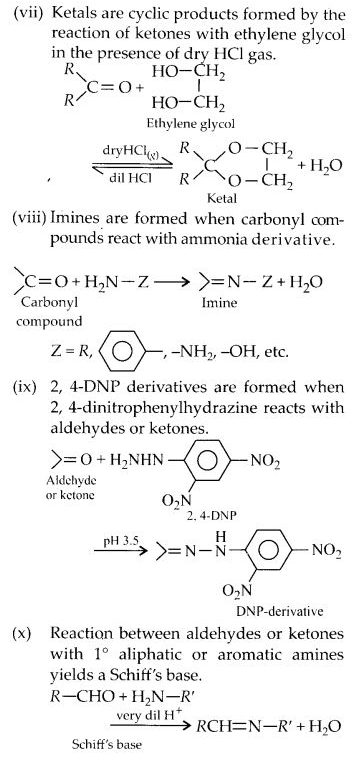
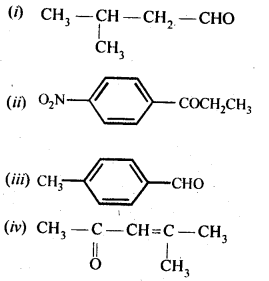
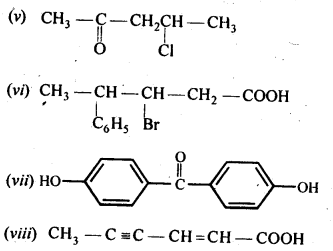
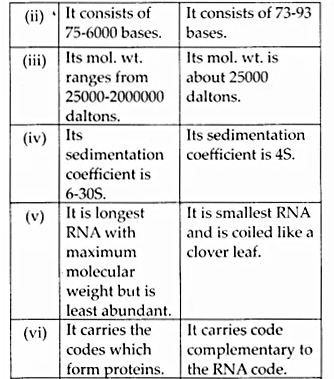
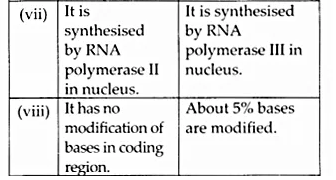
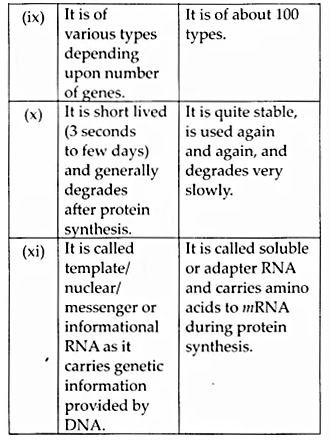
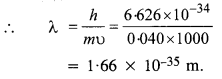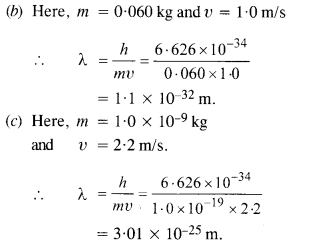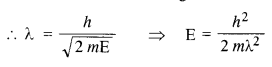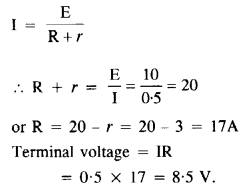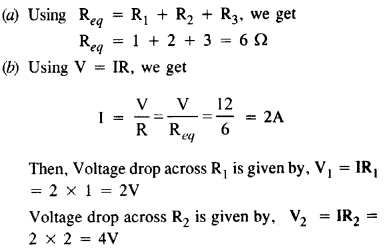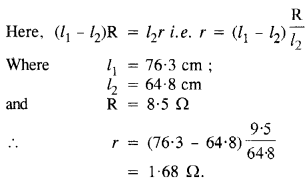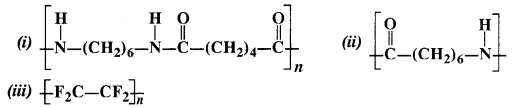Here we are providing NCERT Solutions for Class 12 English Vistas Chapter 8 Memories of Childhood. Students can get Class 12 English Memories of Childhood NCERT Solutions, Questions and Answers designed by subject expert teachers.
Memories of Childhood NCERT Solutions for Class 12 English Vistas Chapter 8
Memories of Childhood NCERT Text Book Questions and Answers
Memories of Childhood Reading with insight
Question 1.
The two accounts that you read above are based on two distant cultures. What is the commonality of theme found in both of them?
Answer:
Both the autobiographical extracts, based upon two distant cultures, depict the lives of two women from marginalised communities who look back at their childhood and reflect on their relationship with mainstream culture. The first account is by an American Indian woman bom in the late 19th century. The account expressed the indignations suffered by the Native Americans at the hands of Christians.
She was ardently against the oppression of Native Americans in Western culture. Though she resented this mistreatment, she still aimed at bridging the wide gap between the dominant white and Native American cultures. She did not let herself get seduced into believing that her Native American traditions were folly or sin.
As a person of mixed blood, her life could be looked upon as an example of the beauty and accomplishments that are possible if the two cultures live in cooperation.The second account is by a contemporary Tamil Dalit writer. She voiced the discrimination she faced as a Dalit. She explored the impact of the discrimination, compounded by the poverty suffered by Dalit women.
The caste system had been so deeply ingrained in the Indian psyche that institutions that ought to promote egalitarianism became the means of perpetuating caste discrimination. Both these accounts are bound by the common theme of discrimination and indignity suffered by women in marginalised communities at the hands of the supposedly superior caste or culture.
Question 2.
It may take a long time for oppression to be removed, but the seeds of rebellion are sowed early in life. Do you agree that injustice in any form cannot escape being noticed even by children?
Answer:
Children are heavily influenced and conditioned by their upbringing. The social influences that they experience from childhood are often ingrained in their subconscious, and are manifested later. Children are also perceptive and keenly aware of positive and negative influences. Rebellious children manifest the ‘seed’ of rebellion planted at some point in their lives.
A sensitive perspective provides a means for understanding how the oppression of children occurs within multiple social contexts that interrelate to produce harmful outcomes for children. Children lack power and resources and are easy targets for adult oppression. Children are exposed to different levels and types of oppression that vary depending on their age, socio-economic class, race, and the beliefs of their parents.
According to the theory of differential oppression, oppression leads to adaptive reactions by children: passive acceptance, exercise of illegitimate coercive power, manipulation of one’s peers, and retaliation. Reducing the oppressive acts of adults and alleviating the damaging circumstances that characterise the social environment of children is critical to reducing the prevalence of juvenile delinquency and other kinds of problem behaviour. The reaction of Zitkala-Sa and Bama to the injustice they perceived as children ranged from defiance, anguish, resentment, and dejection to a fierce determination to excel.
Question 3.
Bama’s experience is that of a victim of the caste system. What kind of discrimination does Zitkala-Sa’s experience depict? What are their responses to their respective situations?
Answer:
Zitkala-Sa was ill-treated and discriminated against from the beginning of her journey. Native Americans, at that time, did not associate with white people and faced discrimination because of their appearance. She felt scared and extremely uncomfortable when unable to comprehend the ways of a foreign culture. Even though she hated the way she was treated, she still had to abide by rules and orders to avoid punishment. However much she suffered at the hands of cultural and racial discrimination, she managed to work her way through and never gave in to discrimination.
Similarly, Bama remained undeterred. She was convinced that she had a role to play. And it was this conviction that made her stand up for her beliefs. She championed the economic and social hardships faced by Dalits due to the discrimination by upper-caste people. Both the writers challenged accepted social practices and did not succumb to pressure. They made their voices heard, and brought to light the indignities faced by their races; they stood by what they believed to be true.
Memories of Childhood Extra Questions and Answers
Memories of Childhood Short Answer Questions
Question 1.
Who was Gertrude Simmons?
Answer:
Gertrude Simmons was an extraordinarily talented and educated Native American woman who struggled and triumphed at a time when severe prejudice prevailed against Native American culture and women. As a writer, she adopted the pen name, Zitkala-Sa. Her works criticized traditional dogma, and her life as a Native American woman was dedicated against the evils of oppression.
Question 2.
What were the first things that upset Zitkala-Sa at school?
Answer:
The severe cold weather and the alien surroundings upset Zitkala-Sa. She was neither familiar with the language nor the strict regime of the hostel. She was perturbed by the sound of the large bell with the annoying the clatter of shoes. Her spirit pined for its lost freedom.
Question 3.
How was the attire of the girls in school different from Zitkala-Sa?
Answer:
The girls at school wore stiff shoes and closely fitted dresses. The small girls wore sleeved aprons and shingled hair. Zitkala-Sa wore soft moccasins, and had wrapped a blanket on her shoulders. She found the tight-fitting clothes rather immodest.
Question 4.
Narrate Zitkala-Sa’s embarrassment at the breakfast table.
Answer:
At the breakfast table, a small bell was tapped, and each of the pupils drew a chair from under the table. Supposing this act meant that they were to be seated, Zitkala-Sa pulled out her chair and seated herself only to realize that the rest were standing. Just as she got up, a second bell was sounded. All were seated at that, and Zitkala-Sa had to crawl back into her chair again. She then heard a man’s voice and saw all had hung their heads over their plates. She saw a pale-faced woman stare at her and dropped her eyes. With the third bell, everyone picked up their knives and forks and began eating. Zitkala-Sa wept as she was confounded by the unfamiliarity of her surroundings and practices.
Question 5.
Why was cutting of her hair the greatest blow to Zitkala-Sa?
Answer:
Zitkala-Sa had been taught by her mother that, in Native American culture, unskilled warriors who were captured had their hair shingled by the enemy. Short hair was worn by mourners, and shingled hair by cowards according to their practices. She tried to resist being shorn of her cultural values.
Question 6.
What were the sights that fascinated little Bama as she walked home from school?
Answer:
Bama enjoyed the entertaining novelties and oddities on the streets, on her way home from school. The performing monkey, the antics of the snake-charmer, the cyclist at his cycle for three days, the Maariyaata temple and the huge bell, the pongal offerings being cooked, the dried fish stall, the sweet stall, the stall selling fried snacks, the street light changing colour, and other such sights fascinated her.
Question 7.
What was that one episode that left an indelible imprint on Bama’s mind?
Answer:
Bama noticed a man holding on to a food packet by its string. Without touching it, he handed it over to the landlord. The landlord opened the parcel and began to eat the vadais. At home, her elder brother explained to how lower cast people, such as the author herself, were prohibited to touch anything of use to the upper caste people. They were believed to sully the item they touched and therefore the package was carefully carried, by its string, for the landlord who belonged to higher caste.
Question 8.
What was the bitter truth of their life that Annan told Bama?
Answer:
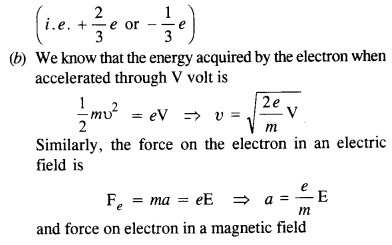
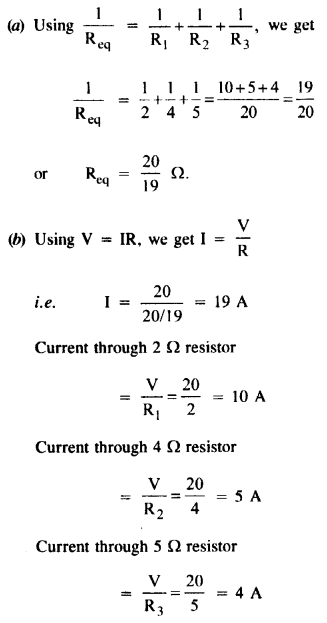
Annan told Bama about the caste system that subjugated Dalits in the society. He added that being bom into a lower caste community, one could never get honour or dignity or respect. It was education alone that could help them earn respect.
Memories of Childhood Long Answer Questions
Question 1.
During her first meal in the missionary school, Simmons became painfully aware of the tension between tradition and acculturation and of the great lack of understanding people had about Native American culture. Explain.
Answer:
Before the meal, the girls were lined up. The Indian girls wore stiff shoes and closely clinging dresses, which seemed immodest to the author’s traditional taste. The small girls wore short hair. Simmons walked noiselessly in her soft leather shoes. She was embarrassed as her blanket had been taken off from her shoulders. Just as they entered, the boys came in from the opposite door. She was at a loss when the rest of them prayed, and ate with fork and knife, by the summons of bells.
She noticed the pale-faced woman staring at her and felt unnerved. The writer wept, she was at a loss over the strange practices, she lacked understanding of the western way of dressing, praying and eating. For a child, a sudden change of one’s faith, cultural and aesthetic conditioning is bewildering and often traumatic. The distance between the two cultures is pronounced by the example of the episode. What the west considered barbaric in Native American was the essence and beauty of their culture which was painfully and ignorantly stripped from their personality in an attempt to acclimatize them.
Question 2.
Narrate the trauma Simmons faced as a child during the hair-cutting episode.
Answer:
Judewin, who knew a few words of English, told Gertrude Simmons that they would cut their long, heavy hair. It was a blow to her as in their culture it signified humiliation or mourning. Judewin warned her about the futility of her mission to escape the hair-cutting ritual, but the writer was prepared to struggle. To hide from the situation, she crept up the stairs into a dim, large room, and crawled under the farthest bed in the dark comer. Every time footsteps were heard, she felt terrorized.
She knew that they were searching frantically for her and soon she was dragged out and tied to a chair. She wept loudly but her hair was clipped short. With her hair lost, she lost her spirit. She had suffered a great deal of disgrace earlier and now her hair was cut like that of a coward. She moaned for her mother, but no one came to comfort her. The brutality made her feel like a beast.
Question 3.
Bama like any other child enjoyed innocent pleasures. Justify.
Answer:
The writer walked home from school each day. It was a ten-minute walk but it took her thirty minutes to complete the distance. She would loiter along, watching all the fun and games and all the entertaining new and strange things that she saw on the way. She enjoyed everything, be it the performing monkey, or the snake charmer, or the man who cycled non-stop for days or the spinning wheels. She was fascinated by the Maariyaata temple, its huge bell and the pongal offerings.
She also stopped to hear the political activists, rallying, or to watch a street play, a puppet show, or a stunt performance.Even mundane activities such as waiters cooling coffee, or people chopping onions, or the almond tree with its fruit occasionally blown down held her attention for long. The people selling edibles were equally appealing to her.
Question 4.
Only an untouchable would know the pain of being one. Discuss with reference to Bama’s episode.
OR
How does “Memories of Childhood” bring out the plight of marginalized communities in India?
Answer:
Bama felt and experienced untouchability, early in life. As a child, she noticed an elderly person walking with a small packet, holding on to the packet by its string, without touching it. He brought it to the landlord, bowed low, and extended the packet towards him. The landlord opened the parcel and began to eat the vadais. Her elder brother explained that people believed that they were upper caste and therefore prohibited people from the lower caste to touch them, believing that the act would pollute them.
On hearing that, Bama felt sad and insulted. Her mind rebelled against fetching and carrying for people who considered people such as her untouchable. It made her very angry. She wondered why the upper-caste people behaved in such a manner just because they were richer. She strongly felt that her people must never run errands for those of the upper caste people.