NCERT Solutions for Class 7 Science Chapter 10 Respiration in Organisms are part of NCERT Solutions for Class 7 Science. Here we have given NCERT Solutions for Class 7 Science Chapter 10 Respiration in Organisms.
| Board | CBSE |
| Textbook | NCERT |
| Class | Class 7 |
| Subject | Science |
| Chapter | Chapter 10 |
| Chapter Name | Respiration in Organisms |
| Number of Questions Solved | 9 |
| Category | NCERT Solutions |
NCERT Solutions for Class 7 Science Chapter 10 Respiration in Organisms
Question 1.
Why does an athlete breathe faster and deeper than usual after finishing the race?
Answer:
During running, the athlete uses a lot of energy. So, he/she needs more energy and breathes faster.
Question 2.
List the similarities and differences between aerobic and anaerobic respiration.
Answer:
Similarities:
| Aerobic Respiration | Anaerobic Respiration |
| 1. It starts with the breakdown of a nutrient (glucose). | 1. It also starts with the breakdown of a nutrient (glucose). |
| 2. It yields byproducts. | 2. It also yields byproducts. |
| 3. It takes place in a cell. | 3. It also takes place in a cell. |
| 4. In this process energy is released. | 4. In this process also energy is released. |
Differences:
| Aerobic Respiration | Anaerobic Respiration |
| 1. It is the process of breakdown of glucose in the presence of oxygen. | 1. It is the process of breakdown of glucose in the absence of oxygen. |
| 2. Glucose is completely oxidized. | 2. Glucose is incompletely oxidized. |
| 3. The end products formed are CO2, H2O, and energy. | 3. The end products formed are C02, ethyl alcohol (organic acid), and energy. |
| 4. Energy released is more. (38 ATP molecules). | 4. Energy released is less (2 ATP molecules). |
| 5. It takes place in all higher organisms. | 5. It takes place in lower organisms like yeast and the muscles of man. |
| 6. The reactions take place in the cytoplasm and mitochondria. | 6. The reactions take place only in the cytoplasm. |
Question 3.
Why do we often sneeze when we inhale a lot of dust-laden air?
Answer:
The air around us has various types of unwanted particles, such as smoke, dust, pollens, etc. When we inhale, the particles get trapped in the hair present in our nasal cavity. However, sometimes these particles may get past the hair in thç nasal cavity. Then they irritate the lining of the cavity, as a result of which we sneeze. Sneezing expels these foreign particles from the inhaled air and dust-free, clean air enters our body.
Question 4.
Take three test-tubes. Fill 3/4th of each with water. Label them A, B and C. Keep a snail in test-tube A, a water plant in test-tube B, and in C keep snail and plant both. Which test-tube would have the highest concentration of CO2?
Answer:
The exchange of gases in three test-tubes can be shown as in Fig.
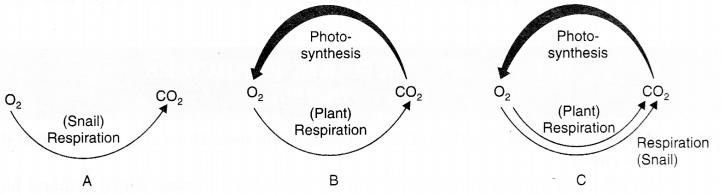
Fig. (A) In test tube A; CO2 is produced by the respiration of snail (No consumption of CO2).
(B) In test tube B; CO2 is produced by the respiration of the plant and a much higher amount of CO2 is consumed during photosynthesis.
(C) In test tube C; CO2 is produced by the respiration of snail and plant and CO2 is consumed during photosynthesis.
It is clear from the above observation that the highest concentration of CO2 will be in test tube A.
Question 5.
Tick the correct answer:
(a) In cockroaches, air enters the body through
(i) lungs
(ii) gills
(iii) spiracles
(iv) skin
(b) During heavy exercise, we get cramps in the legs due to the accumulation of
(i) carbon dioxide
(ii) lactic acid
(iii) alcohol
(iv) water
(c) Normal range of breathing rate per minute in an average adult person at rest is:
(i) 9-12
(ii) 15-18
(iii) 21-24
(iv) 30-33
(d) During exhalation, the ribs
(i) move outwards
(ii) move downwards
(iii) move upwards
(iv) do not move at all
Answer:
(a) (iii) spiracles
(b) (ii) lactic acid
(c) (ii) 15 – 18
(d) (ii) move downwards
Question 6.
Match the items in Column I with those in Column II :
| Column I | Column II |
| (a) Yeast | (i) Earthworm |
| (b) Diaphragm | (ii) Gills |
| (c) Skin | (iii) Alcohol |
| (d) Leaves | (iv) Chest cavity |
| (e) Fish | (v) Stomata |
| (f) Frog | (vi) Lungs and skin |
| (vii) Tracheae |
Answer:
| Column I | Column II |
| (a) Yeast | (iii) Alcohol |
| (b) Diaphragm | (iv) Chest cavity |
| (c) Skin | (i) Earthworm |
| (d) Leaves | (v) Stomata |
| (e) Fish | (ii) Gills |
| (f) Frog | (vi) Lungs and skin |
Question 7.
Mark ‘T’ if the statement is true and ‘F’ if it is false :
During heavy exercise, the breathing rate of a person slows down. (T/F)
Plants carry out photosynthesis only during the day and respiration only at night. (T/F)
Frogs breathe through their skins as well as their lungs. (T/F)
The fishes have lungs for respiration. (T/F)
The size of the chest cavity increases during inhalation. (T/F)
Answer:
(i) F
(ii) F
(iii) T
(iv) F
(v) T
Question 8.
Given below is a square of letters in which are hidden different words related to respiration in organisms. These words may be present in any direction—upwards, downwards, or along the diagonals. Find the words for your respiratory system. Clues about those words are given below the square.
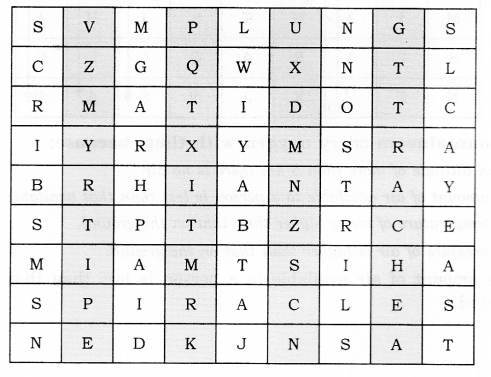
(i) The air tubes of insects
(ii) Skeletal structures surrounding the chest cavity
(iii) Muscular floor of the chest cavity
(iv) Tiny pores on the surface of the leaf
(v) Small openings on the sides of the body of an insect
(vi) The respiratory organs of human beings
(vii) The openings through which we inhale
(viii) An anaerobic organism
(ix) An organism with a tracheal system
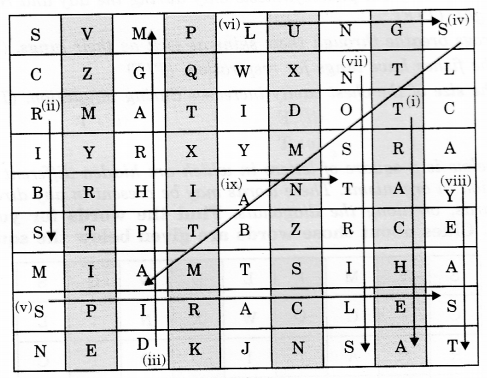
Answer:
(i) TRACHEA
(ii) RIBS
(iii) DIAPHRAGM
(iv) STOMATA
(v) SPIRACLES
(vi) LUNGS
(vii) NOSTRILS
(viii) YEAST
(ix) ANT
These names are indicated by arrows and their serial number are indicated at starting point of the arrow.
Question 9.
The mountaineers carry oxygen with them because:
(a) at an altitude of more than 5 km, there is no air.
(b) the amount of air available to a person is less than that available on the ground.
(c) the temperature of the air is higher than that on the ground.
(d) the pressure of air is higher than that on the ground.
Answer:
(b) The amount of air available to a person is less than that available on the ground.
We hope the NCERT Solutions for Class 7 Science Chapter 10 Respiration in Organisms help you. If you have any query regarding NCERT Solutions for Class 7 Science Chapter 10 Respiration in Organisms, drop a comment below and we will get back to you at the earliest.