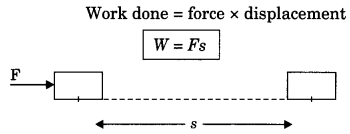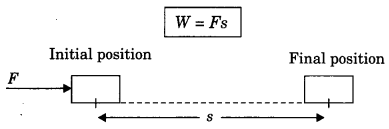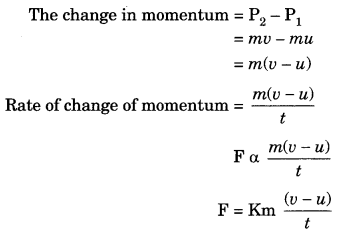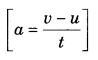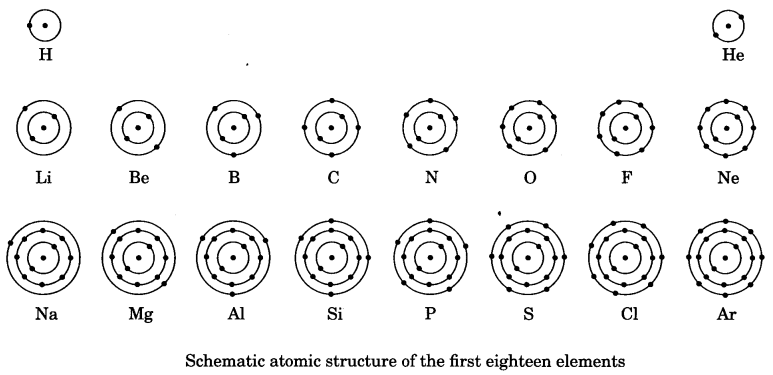
On this page, you will find Sound Class 9 Notes Science Chapter 12 Pdf free download. CBSE NCERT Class 9 Science Notes Chapter 12 Sound will seemingly help them to revise the important concepts in less time.
CBSE Class 9 Science Chapter 12 Notes Sound
Sound Class 9 Notes Understanding the Lesson
1. Sound
Sound is a form of energy which produces a sensation of hearing in our ears.
2. Production of sound
Sound is produced by vibration of objects.
- The sound of human voice is produced due to vibration in the vocal chord.
3. Propagation of sound
Sound propagates in the form of longitudinal waves and these waves require material medium to propagate. Hence sound waves are mechanical waves.
- A wave is a disturbance that moves through a medium when the particles of the medium set neighbouring particles into motion.
- The longitudinal wave of sound travels in the form of compression and rarefaction. Compression is the region of high pressure and rarefaction is the region of low pressure.
- The propagation of sound can be visualised as propagation of density variations or pressure variation in the medium.
4. Sound needs a medium to travel
Sound is a mechanical wave and need a material medium for its propagation.
- Longitudinal wave
A wave in which the particles of the medium oscillate to and fro in the same direction in which the wave is moving is called longitudinal wave. - Transversal wave
A wave motion is said to transverse if the particles of the medium through which the wave propagates vibrate in the direction perpendicular to the direction of propagation of the wave.
5. Some important terms and Relations for longitudinal wave
Frequency: The frequency of wave is defined as the number of waves produced per second.
Or
The frequency of a sound wave is defined as the number of complete oscillations made by the particle of medium in one second.
- It is denoted by greek letter u (nu). Its SI unit is hertz (Hz).
(ii) Wavelength: The distance between two consecutive compressions or two consecutive rarefactions is called wavelength.
- It is usually represented by X (Greek letter lambda). Its SI units is metre (m).
(iii) Time period: The time taken by two consecutive compressions or rarefactions to cross a fixed point is called time period of wave.
Or
- Time taken by particle of medium to complete one oscillation is known as time period.
- It is represented by the symbol T. Its SI unit is second (s).
8. Amplitude: The magnitude of the maximum disturbance in the medium on either side of the mean value is called amplitude of wave. It is usually represented by the letter A. For sound its unit will be that of density or pressure.
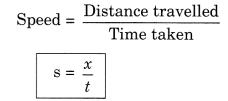
9. Speed: The speed of sound is defined as the distance which a point on a wave, such as a compression or a rarefraction, travels per unit time.
10. Relations
- Relation between time period (T) and frequency (u)
\(T=\frac{1}{v}\) - Relation between speed of wave (υ), wavelength (λ) time period (T) and frequency (υ)
υ = υλ
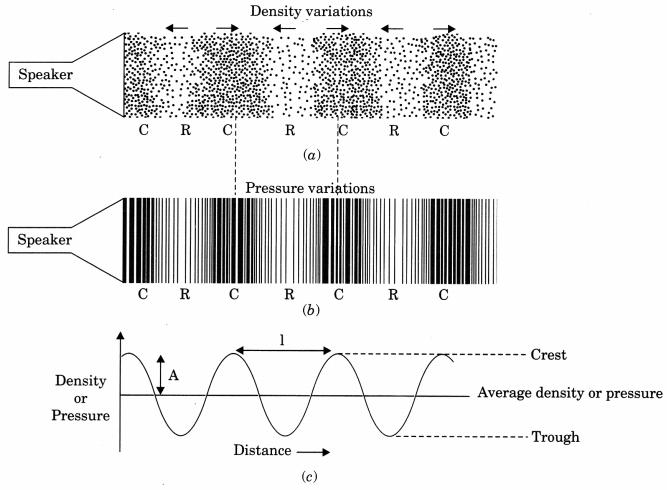
Sound propagates as density or pressure variations as shown in (a), (b) and (c) represents graphically the density and pressure variations.
11. Characteristics of a sound wave
We can describe a sound wave by
- Frequency
- Amplitude
- Speed
12. Frequency-pitch:
- How the brain interprets the frequency of an emitted sound is called its pitch. The faster the vibration of the source, the higher is the frequency and the higher is the pitch.
- The pitch of sound produced by an object of low frequency is low and the source described as flat sound.
- The pitch of sound produced by an object vibrating with high frequency is high and the sound is described as shrill sound.
13. Amplitude-loudness:
- The loudness or softness of a sound is determined by its amplitude. Greater the amplitude of vibration of source, greater is the loudness of sound.
- Loud sound can travel a larger distance as it is associated with high energy.
- A sound wave moves away from the source, its amplitude as well as its loudness decreases.
14. Quality-timber:
- The quality or timber of sound is that characteristics which enables us to distinguish one sound from an¬other having same loudness and pitch.
- A sound of single frequency is called a tone. The sound which is produced due to a mixture of several fre¬quencies is called a note and is pleasant to listen to.
- Quality of sound is represented by waveform.
- Noice is unpleasant to ear. Music is pleasant to hear and is of rich quantity.
15. Speed of sound in different media
- Speed of sound in a medium depends on inertia and elasticity of the medium.
- Speed of sound increases with increase in temperature.
- The speed of sound decreases when we go from solid to gaseous state.
Speed of sound in solids > Speed of sound in liquids > Speed of sound in gases - Speed of sound in air is 331 ms-1 at 0°C and 344 ms-1 at 22°C.
16. Reflection of sound
Sound is reflected in same way as light. Incident and reflected ray make equal angles with the normal to the reflecting surface at the point of incidence and three are in the same plane.
17. Echo
An echo is the phenomenon of repetition of sound by reflection from an obstacle.
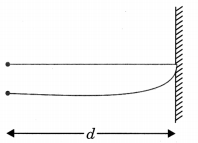
- The sensation of sound lasts in brain for (1/10) of a second. This property is called persistence of hearing. To hear a distinct echo the time interval between the original sound and the reflected one must be at least
0. 1 second. - For hearing a distinct echo, the minimum distance of the obstacle from the source of sound should be 17.2 m.
Take speed of sound = 344 m/s
(at temperature 20°C)
2d = v x t
v = 344 m/s
\(t=\frac{1}{10} \mathrm{s}\)
d=17.2m
18. Reverberation
A sound created in a big hall will persist by repeated reflection from the walls until it is reduced to a value where it is no longer audible. The repeated reflection that results in the persistence of sound is called reveberation.
Excessive reveberation is highly undesirable. To reduce reverberation, the roof and walls of the auditorium are generally covered with sound absorbent materials like compressed fibre board, rough plaster or draperies. The seat materials are also selected on the basis of their sound absorbing properties.
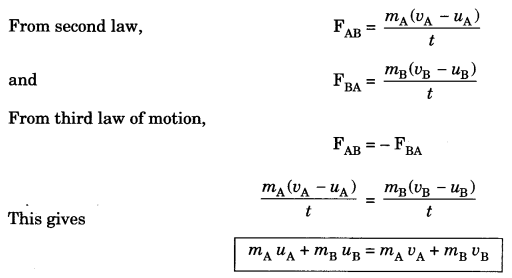
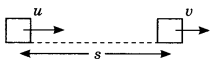
Reflection of sound
d – distance of person from obstacle
v = velocity of sound
t = time after echo is heard
2d = v x t
t = 2 dlv and d = vt/2

19. Uses of multiple reflection of sound
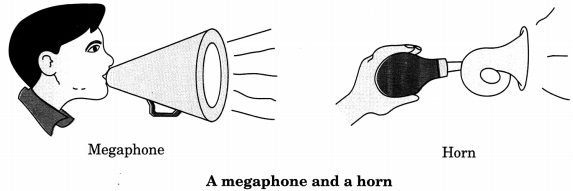
(i) Megaphones, horns, musical instruments such as trumpets and shehnais are all designed to send sound in a particular direction. In these instruments, a tube followed by a conical opening reflects sound successively to guide most of sound successively to guide most of sound from the source in the forward direction towards the audience.
2. Stethoscope is a medical instrument used for listening to sounds produced within the body, chiefly in the heart or lungs
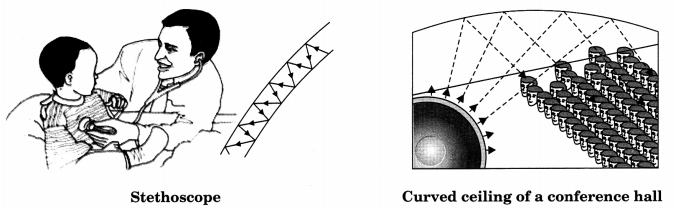
In stethoscopes the sound of the patient’s heartbeat reaches the doctor’s ears by multiple reflection of sound.
3. Generally the ceiling of concert halls, conference halls and cinema halls are curved so that sound after reflection reaches all corners of the hall.
Sometimes curved sound board may be placed behind of the stage so that the sound after reflecting from the sound board, spreads evenly across the width of the hall.
20. Range of Hearing
- Audible range: The sound whose frequency lies between 20 Hz and 20,000 Hz which we are able to hear is called audible sound.
Inaudible range - Infrasonic sound: Sound of frequencies below 20 Hz is called infrasonic sound or infra sound.
- Ultrasonic sound: Frequency higher than 20 KHz is called ultrasonic sound or ultrasound.
21. Uses of Ultrasound in Communication
Sonar
The acronym sonar stands for sound navigation and ranging.
Sonar is a device that uses ultrasonic waves to measure the distance, direction and speed of underwater objects.
22. Components of sonar system
Sonar consists of a transmitter and a detector and is installed in a boat or a ship.
23. Working
The transmitter produces and transmits ultrasound waves. These waves travel through water and after striking the object on the sea bed, get reflected back and are sensed by the detector. The detector converts the ultrasonic waves into electrical signals which are appropriately interpreted.
24. Calculation of distance : The distance of the object that reflected the sound wave can be calculated by knowing the speed of sound in water and the time interval between transmission and reception of the ultrasound
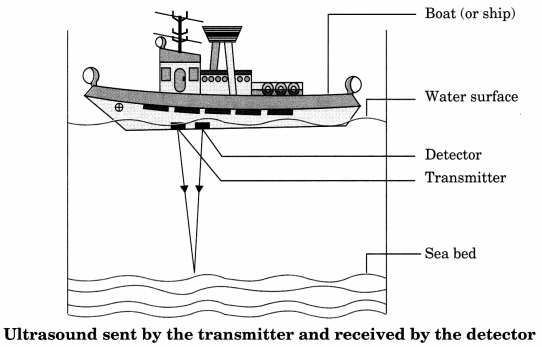
Let the time intervel between transmission and reception of ultrasound signal be t and speed of sound through sea water be υ. The total distance, 2d, travelled by the ultrasound.
- Rhinoceroses communicate using infra sound of frequency as low as 5 Hz.
- Whales and elephants produce sound in infrasonic wave.
- Children under five and some animals, such as dogs can hear infrasonic sound.
Earthquake produces infrasonic waves.
- Ultrasound is produced by dolphines, bats and porpoises. R
- Moths of certain families can hear high frequency waves.
25. Application of Ultrasound
Industrial uses of ultrasound
(i) Cleaning instruments and electronic components
Ultrasound is generally used to clean parts located in hard to reach places, for example, spiral tube, odd shaped parts, electronic component, etc. Objects to be cleaned are placed in a cleaning solution and ultrasonic waves are sent into the solution. Due to the high frequency, the particles of dust, grease and dirt get detached and drop out. The objects are thus thoroughly cleaned.
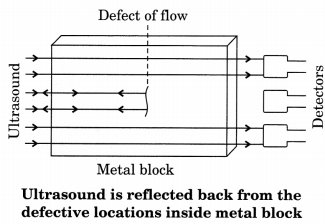
(ii) Detecting flaw and cracks in metal blocks
Ultrasounds can be used to detect crack and flaws in metal blocks. Metallic components are generally used in the construction of big structures like buildings, bridges, machines and also scientific equipments. The cracks or holes inside the metal blocks, which are invisible from outside reduces the strength of the structure. Ultrasonic waves are allowed to pass through the metal block and detectors are used to detect the transmitted waves. If there is even a small defect, the ultrasound gets reflected back indicating the presence of flaws or defect.
Medical uses of ultrasound
(i) Ultrasonography: The technique of obtaining of images of internal organs of the body by using ultrasonic waves is called ultrasonography. An ultrasound scanner is a medical instrument which is used by doctors to detect abnormalities such as stones in gall bladder and kidney or tumours in different organs. In this technique, the ultrasound scanner produces ultrasounds which travel through the tissues of the body, and if there are stones in the gall bladder or kidney or there is tumour in any internal organ, then the ultrasound waves get reflected from these regions due to the change in tissue density. These reflected ultrasound waves are converted into electrical signals and fed to the computer generating a three dimension images of the organ on the monitor of the computer.
(ii) Echocardiography: The technique of obtaining images of the heart by using reflection of ultrasonic waves from various parts of the heart is called echocardiography.
(iii) Breaking of kidney stones: Ultrasound can be used to break small stones formed in the kidney into fine grains. These grains later get flushed out of with urine.
2d = v x t
(iv) Use of ultrasound by bats for determining distance
Bats search out prey and fly in dark night by emitting and detecting reflections of ultrasonic waves. The high pitched ultrasonic squeaks of the bat are reflected from the obstacles or prey and returned to bat’s ear. The nature of reflection tells the bat where the obstacle or pray is and what it is like.
26. Structure of the Human Ear
Introduction
The outer ear is called ‘pinna’. It collects the sound from the surroundings. The collected sound passes through the auditory canal. At the end of the auditory canal there is a thin membrane called the eardrum or tympanic membrane. When compression of the medium reaches the eardrum the pressure on the outside of the membrane increases and forces the eardrum inward.
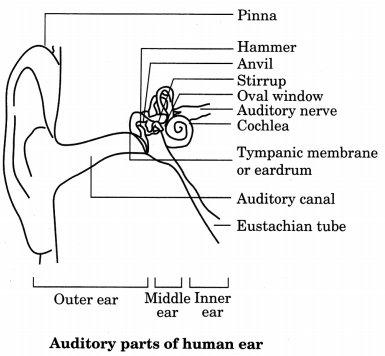
Similarly, the eardrum moves outward when a rarefraction reaches it. In this way the eardrum vibrates. The vibrations are amplified several times by three bones (the hammer, anvil and stirrup) in the middle ear. The middle ear transmits the amplified pressure variations received from the sound wave to the inner ear. In the inner ear, the pressure variations are turned into electrical signals by the cochlea. These electrical signals are sent to the brain via the auditory nerve, and the brain interprets them as sound.
| Parts | Function |
| 1. Pinna | Collects sound from surroundings |
| 2. Hammer, anvil and stirrup | Amplifies vibration or pressure wave |
| 3. Cochlea | Converts pressure variation into electrical signal |
| 4. Eardrum | Thin membrane vibrates when sound reaches inside ear |
| 5. Auditory nerve | Electrical signals are sent to brain from cochlea via auditory nerve. |
Class 9 Science Chapter 12 Notes Important Terms
Sound: Sound is a form of energy which produces a sensation of hearing in our ears.
Longitudinal wave: A wave in which the particles of the medium oscillate to and fro in the same direction in which the wave is moving is called longitudinal wave.
Transverse wave: A wave in which particles of the medium vibrate at right angles to the direction of the propagation of the the wave.
Echo: The repetition of sound caused by the reflection of sound waves is called an echo.
Reverberation: The persistence of sound in a big hall due to repeated reflection of sound from the walls, ceiling and floor of the hall is called reverberation.
Stethoscope: Stethoscope is a medical instrument used for listening to sounds produced within the body, chiefly in the heart or lungs.
Sonar: Sonar is a device that uses ultrasonic wave to measure the distance, direction and speed of underwater object.
ultrasonography: The technique of obtaining images of internal organs of the body by using echoes of ultrasound wave is called ultrasonography.
Echo cardiography: The technique of obtaining images of the heart by using reflection of ultrasonic waves from various parts of the heart is called echo cardiography.