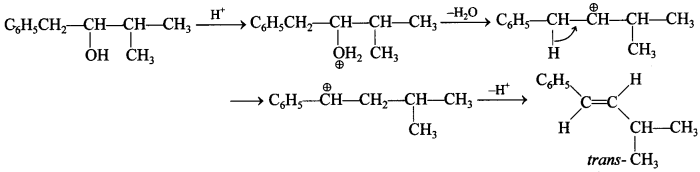
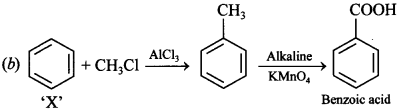
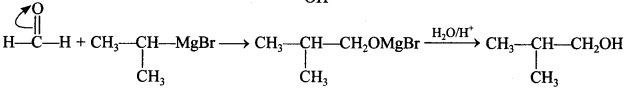
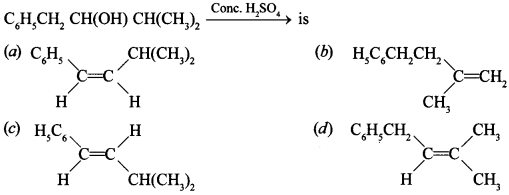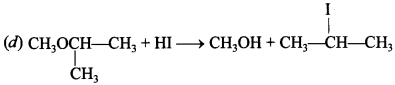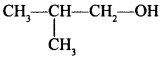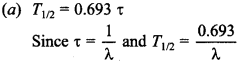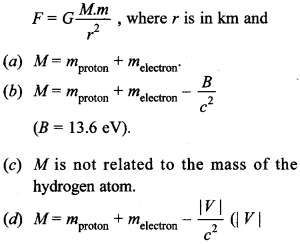Free PDF Download of CBSE Biology Multiple Choice Questions for Class 12 with Answers Chapter 11 Biotechnology: Principles and Processes. Biology MCQs for Class 12 Chapter Wise with Answers PDF Download was Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 12 Biology Biotechnology: Principles and Processes MCQs Pdf with Answers to know their preparation level.
Biotechnology: Principles and Processes Class 12 Biology MCQs Pdf
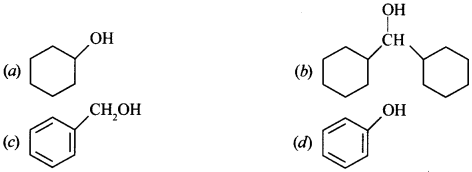
1. Biolistics (gene gun) is suitable for
(a) introducing rDNA into plant cells
(b) introducing rDNA into animal cells
(c) disarming the pathogen vectors
(d) DNA fingerprinting.
Answer
Answer: a
2. In genetic engineering experiments, restriction enzymes are used for
(a) viral DNA
(b) bacterial DNA
(c) eukaryotic DNA
(d) any type of DNA.
Answer
Answer: b
3. The DNA fragments produced by the use of restriction endonucleases can be separated by
(a) polymerase chain reaction
(b) gel electrophoresis
(c) density gradient centrifugation
(d) any of the above.
Answer
Answer: b
4. Plasmids in bacterial cells are
(a) extra-chromosomal DNA, which cannot replicate
(b) extra-chromosomal DNA, which can . self-replicate
(c) extra DNA associated with the genome
(d) extra DNA, associated with the genome, but cannot replicate.
Answer
Answer: b
5. The DNA polymerase enzyme used in PCR is obtained from
(a) Thermus aquaticus
(b) Escherichia coli
(c) Agrobacterium tumefaciens
(d) Salmonella typhimurium.
Answer
Answer: a
6. While isolating DNA from bacteria, which of the following enzymes is not used? [NCERT Exemplar]
(a) Lysozyme
(b) Ribonuclease
(c) Deoxyribonuclease
(d) Protease
Answer
Answer: a
7. Significance of ‘heat shock’ method in bacterial transformation is to facilitate [NCERT Exemplar]
(a) binding of DNA to the cell wall
(b) uptake of DNA through membrane transport proteins
(c) uptake of DNA through transient pores in the bacterial cell wall
(d) expression of antibiotic resistance gene
Answer
Answer: c
8. Which of the following steps are catalysed by Taq polymerase in a PCR reaction? [NCERT Exemplar]
(a) Denaturation of template DNA
(b) Annealing of primers to template DNA
(c) Extension of primer end on the template DNA
(d) All of the above
Answer
Answer: c
9. Which of the given statement is correct in the context of observing DNA separated by agarose gel electrophoresis? [NCERT Exemplar]
(a) DNA can be seen in visible light
(b) DNA can be seen without staining in visible light
(c) Ethidium bromide stained DNA can be seen in visible light
(d) Ethidium bromide stained DNA can be seen under exposure to UV light
Answer
Answer: d
10. ‘Restriction’ in Restriction enzyme refers to [NCERT Exemplar]
(a) cleaving ofphosphodiester bond in DNA by the enzyme
(b) cutting of DNA at specific position only
(c) prevention of the multiplication of bacteriophage in bacteria
(d) all of the above
Answer
Answer: c
11. The role of DNA ligase in the construction of a recombinant DNA molecule is [NCERT Exemplar]
(a) formation of phosphodiester bond between two DNA fragments
(b) formation of hydrogen bonds between sticky ends of DNA fragments
(c) ligation of all purine and pyrimidine bases
(d) none of the above
Answer
Answer: b
12. In an experiment, recombinant DNA bearing ampicillin-resistance gene is transferred into E.coli cells. The host cells are then cultured on a medium containing ampicillin. The result will be
(a) both transformants and non-transformants cannot survive.
(b) both transformants and non-transformants can survive.
(c) transformants only and not the non-transformants can survive.
(d) transformants cannot survive, but non-transformants can not.
Answer
Answer: c
13. The _______ in a vector helps in identifying the transformants and eliminating the non-transformants.
Answer/Explanation
Answer:
Explaination: Selectable marker.
14. When the enzyme _______ is inactivated in E.coli, the transformants do not produce any colour in the presence of a chromogenic substrate.
Answer/Explanation
Answer:
Explaination: β-galactosidase.
15. _______ is the method of bombarding high velocity microparticles of gold or tungsten coated with DNA, into plant cells.
Answer/Explanation
Answer:
Explaination: Biolistics/gene gun.
16. To isolate DNA from fungal cells for biotechnology experiments, enzyme _______ is necessary.
Answer/Explanation
Answer:
Explaination: Chitinase.
17. _______ is used as cloning vector for transformation in plant cells.
Answer/Explanation
Answer:
Explaination: Agmbacterium.
18. Downstream processing involves separation and _______ .
Answer/Explanation
Answer:
Explaination: Purification.
19. _______ is the process of extraction of DNA from the separated bands of DNA in agarose gel.
Answer/Explanation
Answer:
Explaination: Elution.
20. _______ gene in the E.coli vector, pBR 322 codes for enzymes/proteins involved in the replication of the plasmid.
Answer/Explanation
Answer:
Explaination: Rop.
21. _______ are the E.coli enzymes that remove the nucleotides from the ends of DNA strands.
Answer/Explanation
Answer:
Explaination: Exonucleases.
22. _______ is an autonomously replicating, circular, extra-chromosomal DNA in bacterial cells.
Answer/Explanation
Answer:
Explaination: Plasmid.
23. Match the items in Column I with those in Column II.
| Column I | Column II |
| A. Sea weeds | 1. Gel electrophoresis |
| B. Staining of DNA | 2. Source of Agarose |
| C. Separation of DNA fragments | 3. Isolation of DNA from the gel |
| D. Elution | 4. Ethidium bromide |
Answer/Explanation
Answer:
Explaination: A – 2, B – 4, C – 1, D – 3
24. Match the Column l with the Column II.
| Column I | Column II |
| A. Competent host | 1. Separation and purification |
| B. Cloning vector | 2. Culturing of cells in large volumes |
| C. Downstream processing | 3. Taq polymerase |
| D. PCR | 4. Divalent cation (Ca2+) |
| E. Bioreactor | 5. pBR 322 |
| 6. Gel electrophoresis |
Answer/Explanation
Answer:
Explaination: A – 4, B – 5, C – 1, D – 3, E – 2
25. Since DNA fragments are positively charged they move towards the anode. [True/False]
Answer/Explanation
Answer:
Explaination: False.
26. Agarose, the most commonly used matrix in gel electrophoresis is obtained from fungi. [True/False]
Answer/Explanation
Answer:
Explaination: False.
27. The DNA fragments separate according to their size through agarose gel during electrophoresis. [True/False]
Answer/Explanation
Answer:
Explaination: True.
28. The cloning vector pBR 322 has three antibiotic-resistance genes. [True/False]
Answer/Explanation
Answer:
Explaination: False.
29. The vector DNA and foreign DNA are cut by the same restriction endonuclease. [True/False]
Answer/Explanation
Answer:
Explaination: True.
Directions (Q30 to Q32): Mark the odd one in each of the following groups.
30. Cellulose, Lysozyme, Chitinase, Endonuclease
Answer/Explanation
Answer:
Explaination: Endonuclease.
31. Hind III, EcoRI, Sal I, Rop
Answer/Explanation
Answer:
Explaination: Rop.
32. Denaturation, Elution, Annealing, Extension
Answer/Explanation
Answer:
Explaination: Elution.
33. How has European Federation of Biotechnology (EFB) defined biotechnology?
Answer/Explanation
Answer:
Explaination: The European Federation of Biotechnology defines biotechnology as the integration of natural science and organisms, cells or parts thereof and molecular analogues, for products and services; it encompasses both .traditional and modem molecular biology.
34. Name the technique that is used to alter the chemistry of genetic material (DNA/RNA) to obtain the desired result. [Delhi 2016C]
Answer/Explanation
Answer:
Explaination: Genetic engineering.
35. What is meant by gene cloning?
Answer/Explanation
Answer:
Explaination: Gene cloning refers to the process in which a numer of identical copies of a gene of interest are made by introducing the gene into an appropriate host using a suitable Vector.
36. Why is it not possible for an alien DNA to become part of a chromosome anywhere along its length and replicate normally? [AI 2014]
Answer/Explanation
Answer:
Explaination: The alien DNA has to be linked to a specific sequence of DNA, called origin of replication, on the chromosome; this origin of replication is responsible for initiation of replication.
37. Name the specific sequence of DNA in a plasmid that the ‘gene of interest’ ligates with, to enable it to replicate. [AI 2013C]
Answer/Explanation
Answer:
Explaination: Origin of replication (Ori)
38. Write the two components of the first artificial recombinant DNA molecule constructed by Cohen and Boyer. [Foreign 2014]
Answer/Explanation
Answer:
Explaination: An antibiotic-resistance gene and the plasmid of Salmonella typhimurium.
39. Name the two enzymes that are essential for constructing a recombinant DNA. [Delhi 2017C]
Answer/Explanation
Answer:
Explaination: Restriction endonuclease and DNA ligase.
40. In the year 1963, two enzymes responsible for restricting the growth of bacteriophage in E.coli were isolated. How did the enzymes act to restrict the growth of the bacteriophage? [AI 2011C]
Answer/Explanation
Answer:
Explaination:
– One of them (exonucleases) added methyl groups to DNA.
– The other (endonucleases) cut the DNA at specific points within the DNA.
41.Name the enzyme that helps to join DNA fragments. [AI 2017C]
Answer/Explanation
Answer:
Explaination: DNA ligase
42. Mention the role of Restriction enzyme in Recombinant DNA technology. [Delhi 2017C]
Answer/Explanation
Answer:
Explaination: They act as molecular scissors to cut DNA at specific locations.
43. What does ‘R’ stand for, in the restriction endonuclease, EcoRI?
Answer/Explanation
Answer:
Explaination: R stands for RY 13, the strain of E.coli bacterium.
44. A plasmid and a DNA sequence in a cell need to be cut for producing recombinant DNA. Name the enzyme that acts as molecular scissors to cut the DNAs.
Answer/Explanation
Answer:
Explaination: Restriction endonucleases.
45. Suggest a technique to a researcher who needs to separate fragments of DNA. [Delhi 2016]
Answer/Explanation
Answer:
Explaination: Gel electrophoresis.
46. Name the material used as matrix in gel electrophoresis and mention its role. [AI 2014C]
Answer/Explanation
Answer:
Explaination: Agarose; it provides the sieving effect for the DNA to resolve according to their size.
47. Why do DNA-fragments move towards the anode during gel electrophoresis? [CBSE 2018C, Delhi 2011C; HOTS]
Answer/Explanation
Answer:
Explaination: DNA-fragments are negatively charged and hence, move towards the anode.
48. What is the role of ethidium bromide during agarose-gel electrophoresis of DNA fragments?
Answer/Explanation
Answer:
Explaination: The gel is stained by ethidium bromide, to view the separated DNA bands when exposed to UV light.
49. Which main technique and instrument is used to isolate DNA from a plant cell? [CBSE Sample Paper 2013, 2014]
Answer/Explanation
Answer:
Explaination:
– Centrifugation is the technique.
– Centrifuge is the instrument.
50. Name two commonly used vectors for genetic engineering.
Answer/Explanation
Answer:
Explaination: Plasmids and bacteriophages.
51. Why are engineered vectors preferred these days?
Answer/Explanation
Answer:
Explaination:
(i) Engineered vectors facilitate easy linking of foreign DNA.
(ii) They help in selection of recombinants.
52. Mention the uses of cloning vectors in biotechnology. [Delhi 2011]
Answer/Explanation
Answer:
Explaination:
(i) Cloning vectors are used to make multiple copies of the desired DNA/ gene.
(ii) They are used to transfer the gene of interest to the host cell.
53. Why is ‘plasmid’ an important tool in biotechnology experiments? [AI2013C]
Answer/Explanation
Answer:
Explaination: Since plasmids can replicate within the bacterial cell independently of the genomic DNA, any alien DNA ligated to it will also multiply, i.e. it is used as a vector as well as in gene cloning.
54. Why is it essential to have a ‘selectable marker’ in a cloning vector? [AI 2010; HOTS]
Answer/Explanation
Answer:
Explaination: It helps in identifying the recombinants from the non-recombinants.
55. Why are antibiotic-resistance genes used as markers in E.coli? [HOTS]
Answer/Explanation
Answer:
Explaination: It is because the normal E. coli cells do not have resistance to any such antibiotics.
56. Name two antibiotic-resistance genes in the pBR 322 of E.coli plasmid.
Answer/Explanation
Answer:
Explaination: Ampicillin-resistance gene and tetracyclin- resistance gene.
57. A plasmid without a selectable marker was chosen as vector for cloning a gene. How does this affect the experiment? [HOTS]
Answer/Explanation
Answer:
Explaination: In the absence of a selectable marker, it will not be possible to identify the recombinants from the non-recombinants.
58. State what happens when an alien gene is ligated at Sal I site of pBR 322 plasmid. [Delhi 2013C]
Answer/Explanation
Answer:
Explaination: The transformant loses the tetracycline- resistance.
59. State what happens when an alien gene is ligated at Pvu I site of pBR 322 plasmid. [AI 2013C]
Answer/Explanation
Answer:
Explaination: The transformant loses ampicillin-resistance.
60. How is Agrobacterium tumefaciens able to transform a normal plant cell into a tumour? [Delhi 2013C]
Or
Biotechnologists refer to Agrobacterium tumefaciens as a natural genetic engineer of plants. Give reasons to support the statement. [AI 2011]
Answer/Explanation
Answer:
Explaination: When it infects a plant cell, it delivers a part of its DNA, called ‘T-DNA’ (or Tumour- inducing (Ti) plasmid) into the plant cell and transforms it into a tumour cell.
61. How can retroviruses be used efficiently in biotechnology experiments in spite of them being disease causing? [AI 2013C]
Answer/Explanation
Answer:
Explaination: Retroviruses are disarmed (diseases-causing gene is removed/inactivated) and used as vectors to deliver the recombinant/alien DNA into animal cells.
62. Name the commonly used vector for transformation in plant cells.
Answer/Explanation
Answer:
Explaination: Agrobacterium tumefaciens.
63. How does an alien DNA gain entry into a plant cell by ‘biolistic’ method? [Foreign 2013]
Answer/Explanation
Answer:
Explaination: The cells are bombarded with high velocity micro-particles of gold or tungsten, coated with the alien DNA.
64. Mention the type of host cells suitable for the gene guns to introduce an alien DNA. [Delhi 2014]
Answer/Explanation
Answer:
Explaination: Plant cells.
65. Name the host cells in which micro-injection technique is used to introduce an alien DNA. [Foreign 2014]
Answer/Explanation
Answer:
Explaination: Animal cells.
66. Why is the enzyme cellulase needed for isolating genetic material from plant cells and not from animal cells? [Delhi 2013, 2010; HOTS]
Answer/Explanation
Answer:
Explaination: Cellulase is used to digest the cell wall (cellulose) of plant cells; animal cells have no cell wall and hence, it is not needed.
67. How can bacterial DNA be released from the bacterial cell for biotechnology experiments? [Delhi 2011]
Answer/Explanation
Answer:
Explaination: The bacterial cell has to be treated with lysozyme, to digest the cell wall to release DNA.
68. To use the restriction enzyme to cut the DNA, the DNA must be in a pine form, i.e. free from proteins and RNAs associated with it. How is it achieved?
Answer/Explanation
Answer:
Explaination:
– Proteins are removed by using proteases.
– RNAs are removed by using ribonucleases (RNases).
69. Write the names of the enzymes that are used for isolation of DNA from bacterial and fungal cells, respectively for Recombinant DNA technology. [AI, Foreign 2014]
Answer/Explanation
Answer:
Explaination:
– Lysozymes for bacterial cells.
– Chitinase for fungal cells.
70. Write the importance of the bacterium Thermus aquaticus in polymerase chain reaction. [Delhi 2013C]
Answer/Explanation
Answer:
Explaination: It is the source of thermostable DNA- polymerase enzyme.
We hope the given Biology MCQs for Class 12 with Answers Chapter 11 Biotechnology: Principles and Processes will help you. If you have any query regarding CBSE Class 12 Biology Biotechnology: Principles and Processes MCQs Pdf, drop a comment below and we will get back to you at the earliest.











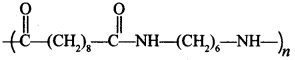






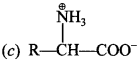


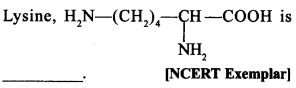




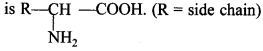
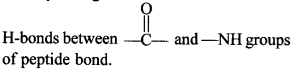

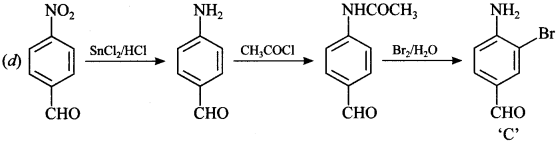
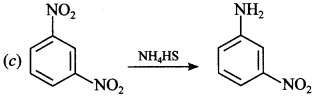




















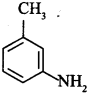



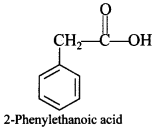
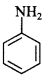 C6H5 group is electron withdrawing which decreases electron density on ‘N’.
C6H5 group is electron withdrawing which decreases electron density on ‘N’.
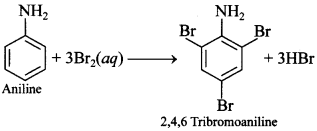









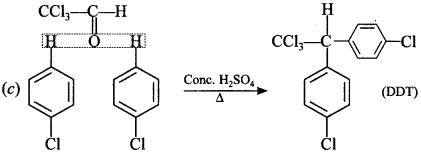

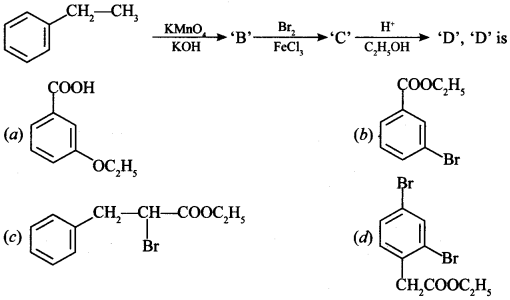

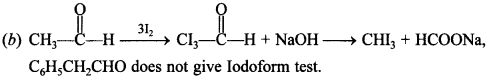



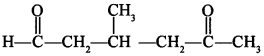

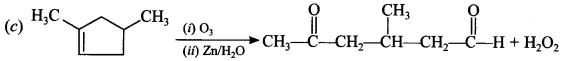













 [True/False]
[True/False]