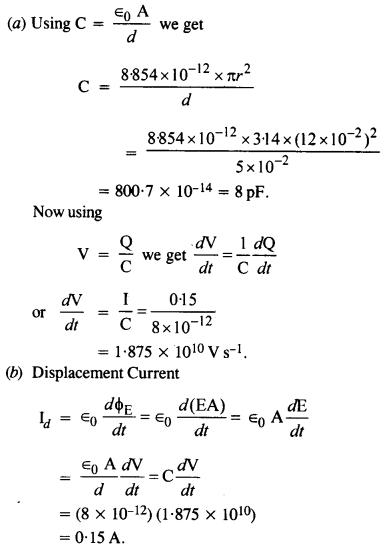
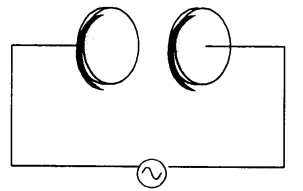
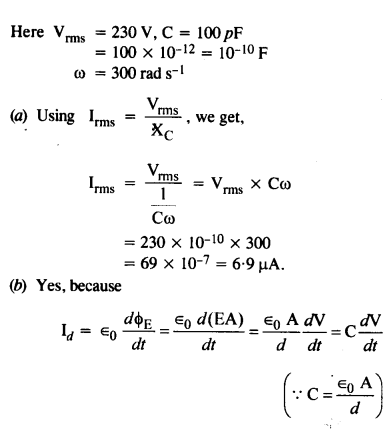
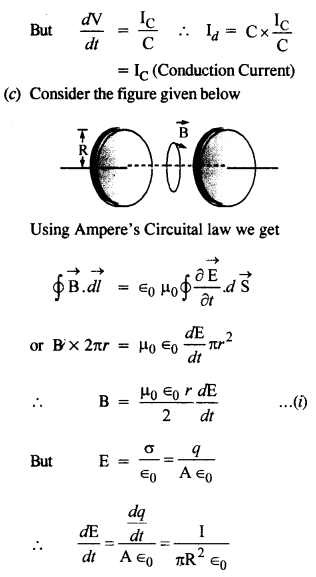
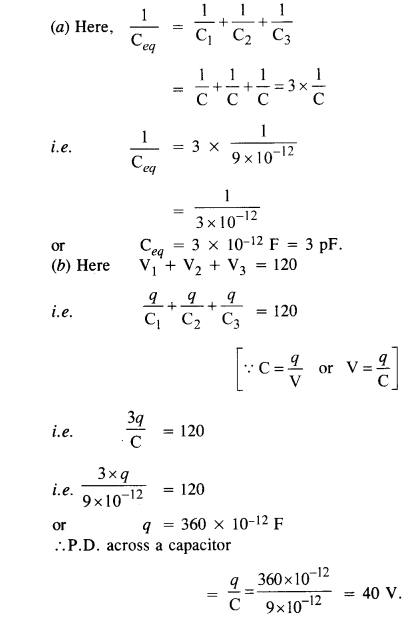
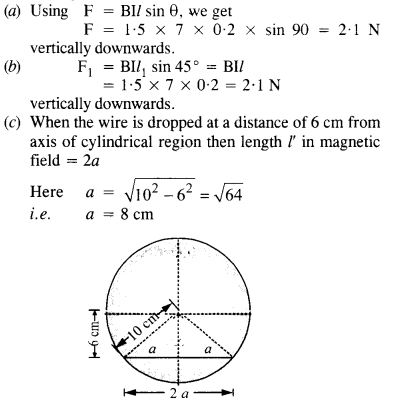
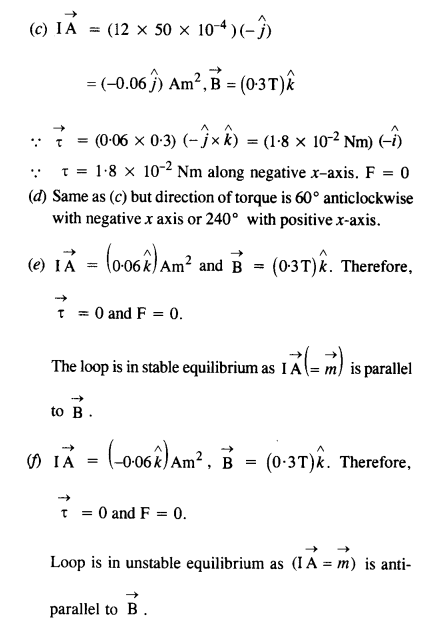
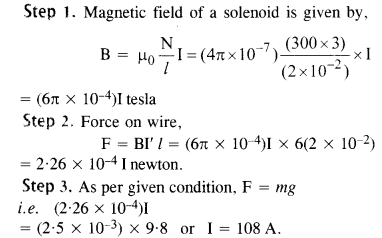
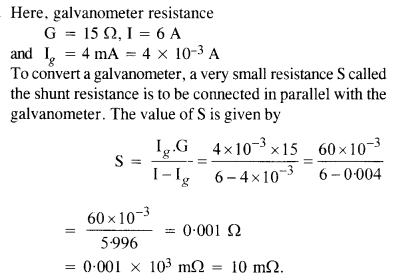
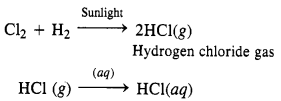
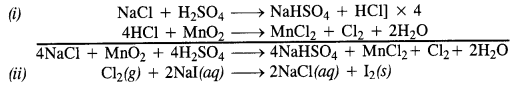
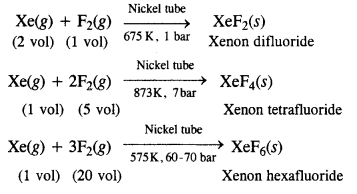
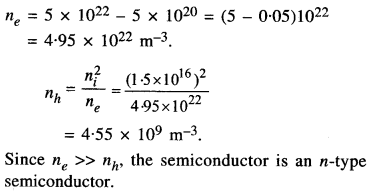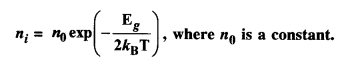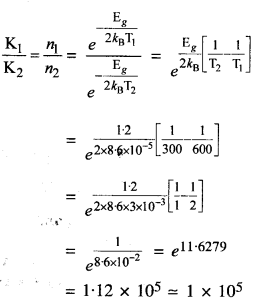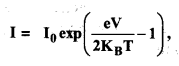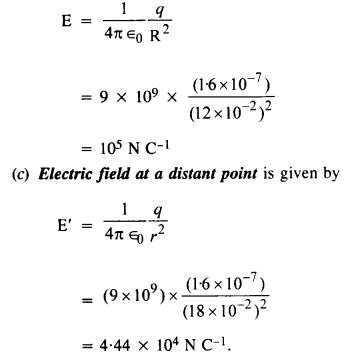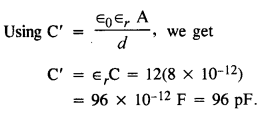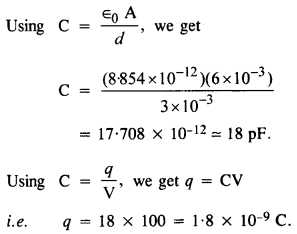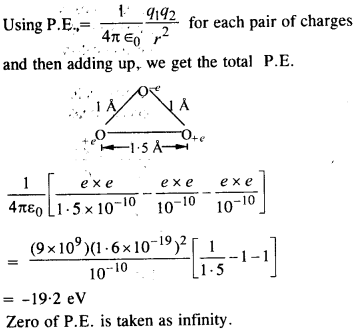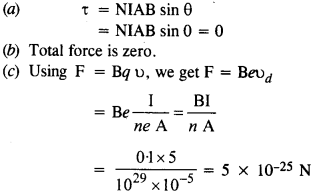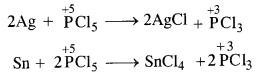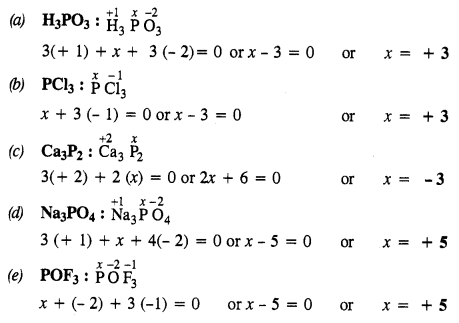NCERT Solutions for Class 12 History Chapter 1 Bricks, Beads and Bones The Harappan Civilisation are part of NCERT Solutions for Class 12 History. Here we have given NCERT Solutions for Class 12 History Chapter 1 Bricks, Beads and Bones The Harappan Civilisation.
| Board | CBSE |
| Textbook | NCERT |
| Class | Class 12 |
| Subject | History |
| Chapter | Chapter 1 |
| Chapter Name | Bricks, Beads and Bones The Harappan Civilisation |
| Number of Questions Solved | 9 |
| Category | NCERT Solutions |
NCERT Solutions for Class 12 History Chapter 1 Bricks, Beads and Bones The Harappan Civilisation
Question 1.
List the items of food available to people in Harappan cities. Identify the groups who would have provided these.
Solution :
(a) The following items of food were available to people in Harappan cities : Wheat, barley, lentil, chickpea, sesame, millets, rice, fish, and goat.
(b)
- Animals such as cattle, sheep, buffalo and pig were domesticated. So, they could get meat from these animals.
- The evidence of a ploughed field at Kalibangan and knowledge of bull prove that harvesting was done by the Harappans.
- Regarding hunting of wild animals such as boar, deer and gharial, there is no proof whether the Harappans hunted these animals themselves or obtained meat from other hunting communities.
Question 2.
How do archaeologists trace socio-economic differences in Harappan society? What are the differences that they notice?
Solution:
Following examples can be cited to show the existence of social and economic variations : in the Harappan society:
(i) Study of burials is one example. In the Harappan sites, the deads were usually laid in pits. There were differences in the Way burial pits were made. At some instances, the hollowed-out spaces were lined with bricks. But these may not be taken as an indication of social differences.
(ii) In some graves pottery and ornaments have been found. Jewellery has been found from the graves of men and women as well. These findings can point out social and economic differences. ‘
(iii) The artefacts have been classified into two categories, Utilitarian and Luxurious. Objects of daily uses and objects made of ordinary materials made of clay or stone come under utilitarian category. Ordinary articles consisted of querns, pottery, flesh-rubbers and needles. These have been found distributed throughout settlements.
(iv) Objects of luxuries were rare and made from precious, non-local materials. The technology used was advanced and complicated. Little pots of faience were considered precious. They were also not easy to make. These show the existence of social and economic variations in the Harappan society.
Question 3.
Would you agree that the drainage system in Harappan cities indicates town planning? Give reasons for your answer.
Solution :
The drainage system in Harappan cities indicates town planning as is clear from the following reasons :
- It was planned drainage system. In the Lower Town, the roads and streets were laid out along an approximate “grid” pattern, intersecting at right angles.
- It seems from the plan of the Lower Town that streets with drains were laid out first and then houses built along them.
- The drains of every house were connected to the street drains. Very long drainage channels were provided at intervals with sumps for cleaning. Drainage system has been found in smaller settlements like Lothal.
Question 4.
List the materials used to make beads in the Harappan civilisation. Describe the process by which any one kind of bead was made.
Solution :
(a) The materials used to make beads in the Harappan civilisation were as given below:
- Stones like carnelian of a beautiful red colour, jasper, crystal, quartz, and steatite;
- Metals like copper, bronze, and gold;
- Shell, faience, and terracotta or burnt clay.
(b)
- The process or technique for making beads differed according to the material.
For example, steatite, a very soft stone, was easily worked. Some beads were moulded out of a paste made with steatite powder. This permitted making a variety of shapes, unlike the geometrical form that were made out of harder stones. - Red colour of carnelian was obtained by firing the yellowish raw material and beads at various stages of production.
- Nodules were chipped into rough shapes, and then finely flaked into the final form.
- Grinding, polishing, and drilling completed the process.
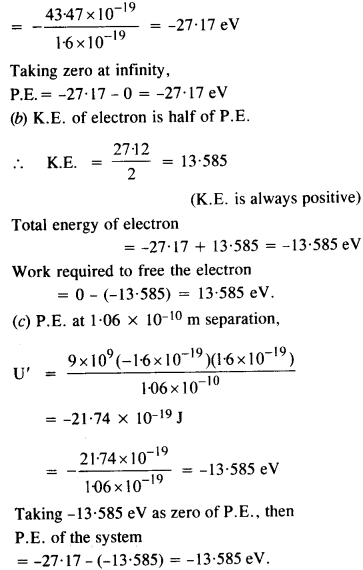
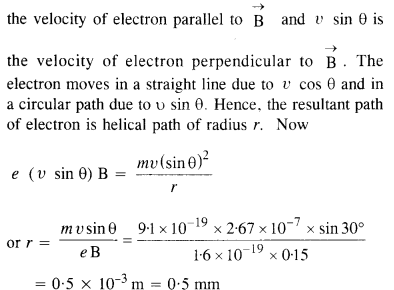
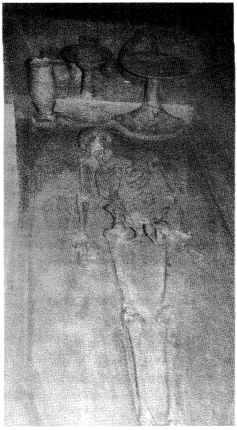
Question 5.
Look at figure and describe what you see. How is the body placed? What are the objects placed near it? Are there any artefacts on the body? Do these indicate the sex of the skeleton?
Solution :
(a) A dead body has been laid in a pit.
(b) Some objects of pottery are placed near it.
(c) There seems to be some ornaments on body but these do not indicate the sex of the skeleton because jewellery has been found in burials of both men and women.
Question 6.
Describe some of the distinctive features of Mohenj odaro.
Or
Describe the features that justify that Mohenjodaro was a planned urban cetnre.
Solution :
Some of the distinctive features of Mohenjodaro were as given below :
- Mohenjodaro is the most well known site. It was divided into two sections – one smaller but higher and the other much larger but lower. These are known as the Citadel and the Lower Town, respectively. Both the sections were walled.
- Several buildings were built on platforms which implies that the building activity was restricted on the platforms. It seems that the settlement was first planned and then implemented accordingly.
- The standardised ratio of bricks – sundried or baked – is also a sign of planning. The length and breadth of bricks were four times and twice the height, respectively.
- There was well-planned drainage system. The roads and streets were laid out along an approximate “grid” pattern, intersecting at right angles. It appears that streets with drains were laid down first and then houses built along them.
- Residential buildings were centered on a courtyard, with rooms on all sides. There were no windows in the walls along the ground level to have privacy. The main entrance too did not give a direct view of the courtyard.
- There was a bathroom in everyhouse. The drains were connected to the street drains.
- Some houses had staircases to reach a second story or the roof.
Question 7.
List the raw materials required for craft production in the Harappan civilisation and discuss how these might have been obtained.
Solution :
Following is the list of materials required for craft production in the Harappan Civilisation:
Stone, clay, copper, tin, bronze, gold, faience, shell, camelian, jasper, crystal, steatite, quartz, timber.
Some of the raw materials were locally available whereas some were purchased from the distant places. Soil and wood were locally available raw materials. Stones, fine quality wood, metals were procured from distant places.
Settlements of the Harappans were situated at such places where raw materials were easily available. Nageshwar and Balacot were famous for shell. Some places were famous for Lapis Lazuli like Shortughai in Afghanistan. Rajasthan and Gujarat were famous for copper. Lothal was famous for camelian.
Another way of obtaining raw material was sending expeditions to different places.
Evidences show that expedition was sent to Khetri region of Rajasthan for copper and to South India for Gold. Through these expeditions local communities were contacted. Harappan evidences found at these places indicate contacts between each other. Evidences found at Khetri region were given the name of Ganeshwar Jodhpura Culture by archaeologists. Huge reserves of copper products were found here. It is assumed that inhabitants of these area sent copper to Harappan people.
Question 8.
Discuss how archaeologists reconstruct the past.
Solution :
It is the material evidence by which the archaeologists reconstruct the past. This material could be pottery, tools, ornaments and household objects. It is done in the following ways:
- Classifying finds : The archaeologists classify their finds in terms of material, such as stone, clay, metal, bone, ivory, etc. and in terms of function i.e., an artefact is a tool or an ornament or both or something meant for ritual use.
- The archaeologists try to reconstruct religious practices because certain objects which seemed unusual or unfamiliar may have had a religious significance. For example, terracotta figurines of women, heavily jewelled, some with elaborate head dresses were regarded as mother goddesses.
- Religious beliefs and practices are reconstructed by examining seals, depicting ritual scenes, animals (one, horned animal) cross-legged yogic figure.
- Many reconstructions are made on the assumption that later traditions provide parallels with earlier ones because archaeologists move from present to the past. The example is ‘proto-Shiva’ seal which can be compared with Rudra mentioned in Rigveda.
Question 9.
Discuss the functions that may have been performed by rulers in Harappan society.
Solution :
The functions that may have been performed by rulers in Harappan society whereas mentioned below –
- There are indications of complex decisions being taken and implemented in Harappan society. For example, the extraordinary uniformity of Harappan artefacts as evident in seals, pottery, weights and bricks would have due the authority of the rulers. A large building has been found in Mohenjodaro. It might be a palace for the rulers. A stone statute has been labelled as ‘priest-king’. Thus, he may be a ruler who exercised authority for taking various decisions.
- Whether the ritual practices were performed by the ‘priest-king’ is not clear because these practices of Harappan civilisation are not well understood yet nor are there any means of knowing whether those who performed them also held political power.
We hope the NCERT Solutions for Class 12 History Chapter 1 Bricks, Beads and Bones The Harappan Civilisation help you. If you have any query regarding NCERT Solutions for Class 12 History Chapter 1 Bricks, Beads and Bones The Harappan Civilisation, drop a comment below and we will get back to you at the earliest.