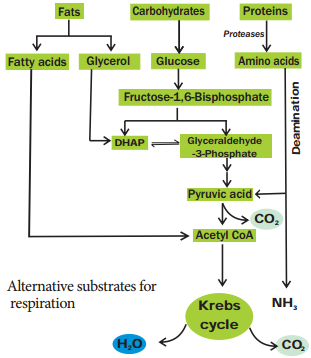
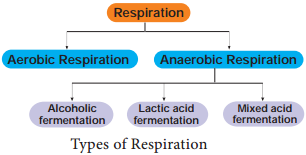
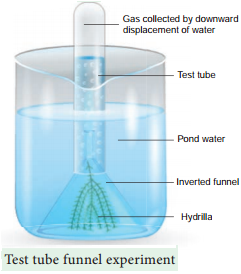
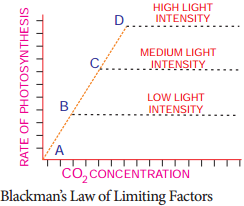
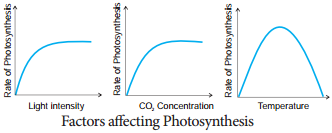
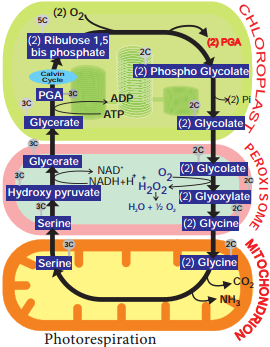
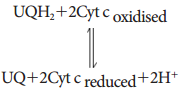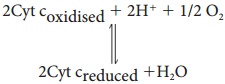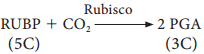Learninsta presents the core concepts of Biology with high-quality research papers and topical review articles.
Respiratory Quotient
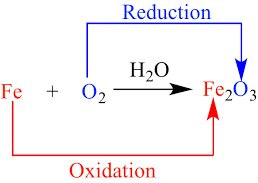
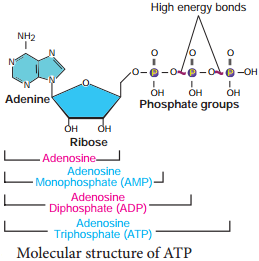
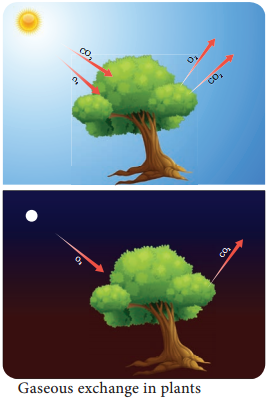
The ratio of volume of carbon dioxide given out and volume of oxygen taken in during respiration is called Respiratory Quotient or Respiratory ratio. RQ value depends upon respiratory substrates and their oxidation.
![]()
1. The respiratory substrate is a carbohydrate, it will be completely oxidised in aerobic respiration and the value of the RQ will be equal to unity.
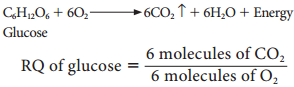
= 1(unity)
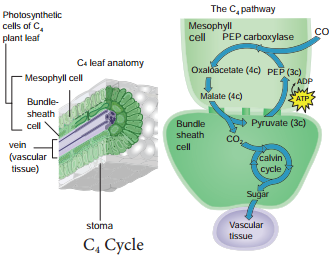
2. If the respiratory substrate is a carbohydrate it will be incompletely oxidised when it goes through anaerobic respiration and the RQ value will be infinity.
![]()
= ∞ (infintiy)
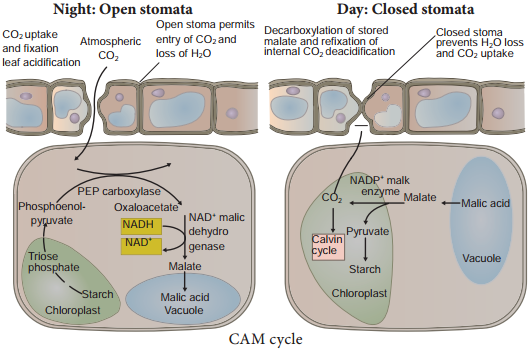
3. In some succulent plants like Opuntia, Bryophyllum carbohydrates are partially oxidised to organic acid, particularly malic acid without corresponding release of CO2 but O2 is consumed hence the RQ value will be zero.
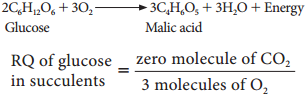
= 0(zero)
4. When respiratory substrate is protein or fat, then RQ will be less than unity.
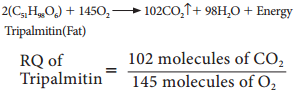
= 0.7 (less than unity)
5. When respiratory substrate is an organic acid the value of RQ will be more than unity.
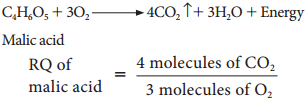
= 1.33 (more than unity)
Significance of RQ
- RQ value indicates which type of respiration occurs in living cells, either aerobic or anaerobic.
- It also helps to know which type of respiratory substrate is involved.