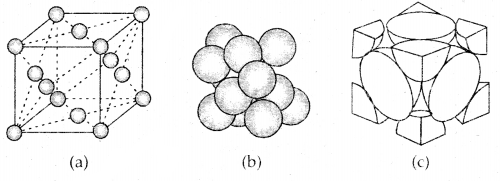
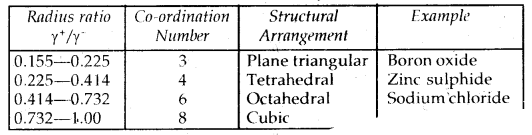
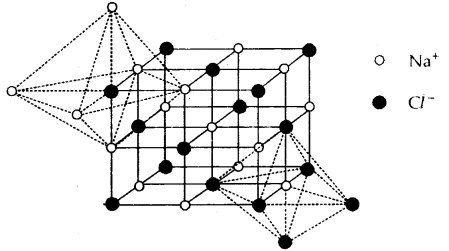
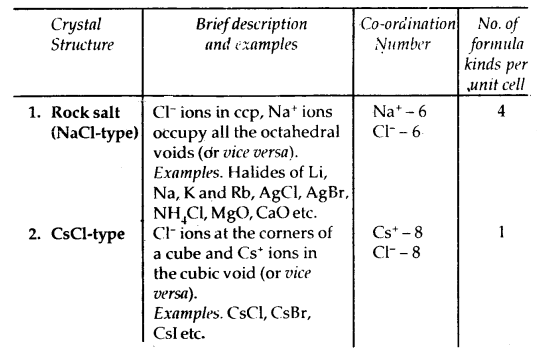
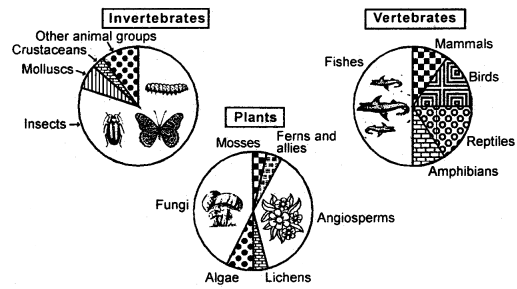
Learninsta presents the core concepts of Microbiology with high-quality research papers and topical review articles.
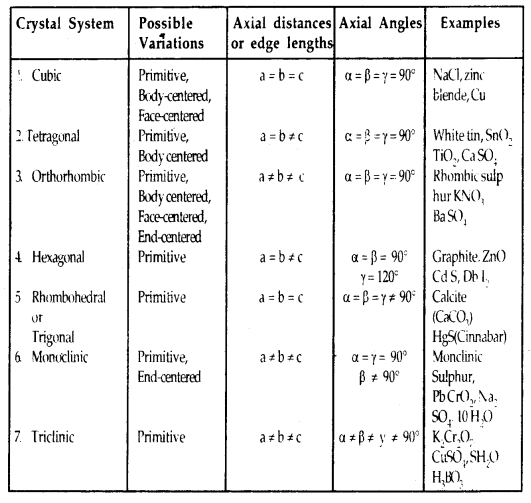
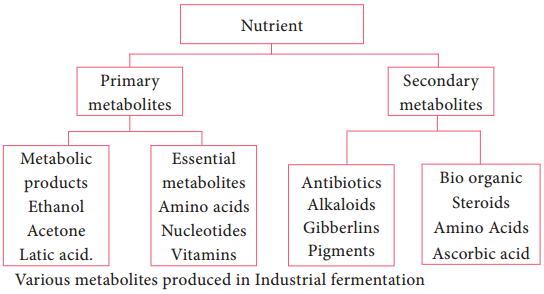
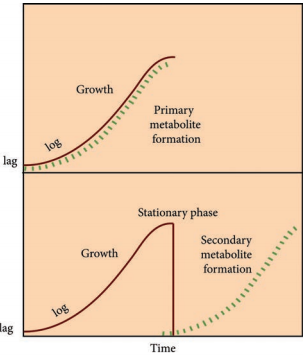
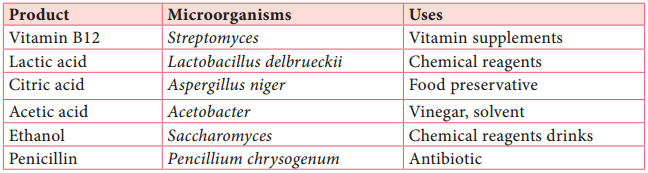
Fermentors of Industrail Microbiology
The main function of a fermenter is to provide a suitable environment in which an organism can efficiently produce a target product. Most of them are designed to maintain high biomass concentrations, which are essential for many fermentation processes.
Fermentor design, quality of construction, mode of operation and the level of sophistication largely depend upon the production organism, the optimal operating conditions required for target product formation, product value and the scale of production.
The performance of any fermenter depends on many factors, but the key physical and chemical parameters that must be controlled are agitation rate, oxygen transfer, pH, and temperature and foam production.
Basic Design of a Fermenter
The materials used for construction of fermenter withstand repeated steam sterilization and are nontoxic. The reaction vessel is designed to withstand vacuum or else it may collapse while cooling. The internal surface is smooth and corrosion resistant. Either stainless steel or glass is used for construction.
Conventional bioreactors are cylindrical vessels with dome top and bottom (Figure 6.5).
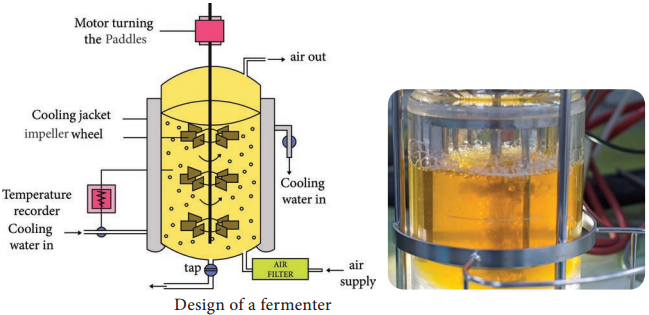
It is surrounded by a jacket and sparger at the bottom through which air is introduced. The agitator (for mixing of cells and medium) shaft is connected to a motor at the bottom. It has ports for pH, temperature, dissolved Oxygen sensors for regulation. Antifoam agents like animal vegetable oil, lard oil, corn oil and soya bean oil are used to control the foam.
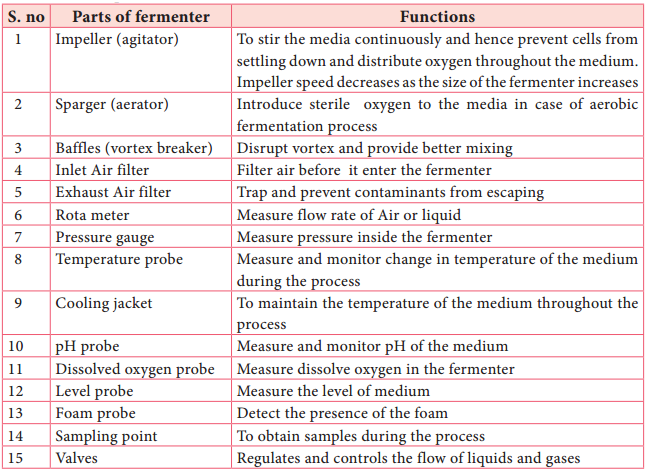
Modern fermentors are usually integrated with computers for efficient process monitoring and data acquisition. Parts of the fermenter and their functions are given in Table 6.2.
Components of fermenter and their uses:
Media Used in the Industrial Productions
Fermentation Medium Most fermentation requires liquid media, often referred to as broth, although some solid-substrate fermentations are also operated. Fermentation media must satisfy all the nutritional requirements of the microorganism and fulfill the technical objectives of the process.
Animal fats and plant oils are also incorporated into some media, often as supplements to the main carbon source. Medium used for large scale production should have the following characteristics.
- It should be cheap and easily available.
- It should maximize the growth of the microorganism productivity and the rate of formation of the desired product.
- It should minimize the formation of undesired products.
It should contain carbon source, nitrogen source, energy source, micro nutrients required for the industrial production. Table 6.3 shows common substances used in the industrial fermentation process.
Waste products from other industrial processes such as molasses, ligno cellulosic waste, and corn steep liquor are generally used as substrates for industrial fermentation.
Apart from carbon and nitrogen sources, some other components like minerals, vitamins, growth factors are also used in Industrial fermentations.
Minerals
Normally, sufficient quantities of cobalt, copper, iron, manganese, molybdenum, and zinc are present in the water supplies, and as impurities in other media ingredients. For example, corn steep liquor contains a wide range of minerals that will usually satisfy the minor and trace mineral needs.
Vitamins and growth factors
Many bacteria can synthesize all necessary vitamins from basic elements. For other bacteria, filamentous fungi and yeasts, they must be added as supplements to the fermentation medium. Most natural carbon and nitrogen sources also contain at least some of the required vitamins as minor contaminants. Other necessary growth factors, amino acids, nucleotides, fatty acids and sterols, are added either in pure form or, for economic reasons, as less expensive plant and animal extracts.
Precursors
Some fermentation must be supplemented with specific precursors, notably for secondary metabolite production. When required, they are often added in controlled quantities and in a relatively pure form. Examples: Phenylacetic acid or phenylacetamide added as side chain precursors in penicillin production.
Large Scale Production
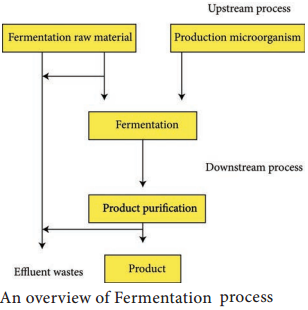
Basic Steps of Industrial Fermentation
Successful development of a fermentation process and fermentors requires major contributions from a wide range of other disciplines, particularly biochemistry, genetics, molecular biology, chemistry, chemical engineering and process engineering, mathematics and computer technology. A typical operation involves both upstream processing (USP) and downstream processing (DSP) stages (Figure 6.6).
Upstream Processing
It is the first step in which biomolecules like bacteria or other cells are grown in a fermentor. Upstream processing involves inoculation development, scale up, medium preparation and sterilization of media and fermentation process.
Inoculum development
It is a preparation of a population of micro organisms from a stock dormant culture to a state useful for inoculating a final production fermentor. It is a critical stage in fermentation process. It is a stepwise sequence employing increasing volume of media. Inoculum media is usually balanced for rapid cell growth and not for product formation.
Inoculum scale up
It is the preparations of the seed culture in amounts sufficient to be used in the larger fermenter vessel. It involves growing the microorganisms obtained from the pure stock culture in several consecutive fermenter. By doing this, the time required for the growth of microbes in the fermenter is cut down, so that the rate of productivity is increased. The seed culture obtained is then used for inoculation in fermentation medium. The size of the inoculums is generally 1 – 10% of the total volume of the medium.
In general, fermentation/bioprocess techniques are developed in stages starting from a laboratory and finally leading to an industry. The phenomenon of developing industrial fermentation process in stages is referred to as scale-up. Scale-up is necessary for implementing new fermentation technique developed using mutant organisms.
The very purpose of scale-up is to develop optimal environmental and operating conditions at different levels for a successful fermentation industry where conditions like substrate concentration agitation and mixing, aeration, power consumption and rate of Oxygen transfer are studied. In a conventional scale-up, a fermentation technique is developed in 3-4 stages.
The initial stage involves a screening process using Petri dishes or Erlenmeyer flasks followed by a pilot project to determine the optimal operating conditions for a fermentation process with a capacity of 5-200 litres. The final stage involves the transfer of technology developed in the laboratory to industry. (Figure 6.7)
It has to be continuously noted that a fermentation process that works well at the laboratory scale may work poorly or may not work at all on industrial scale. Therefore it is not always possible to blindly apply the laboratory conditions of a fermentation technique developed to industry. At the laboratory scale, one is interested in the maximum yield of the product for unit time.
At the industry level, besides the product yield, minimal operating cost is another important factor for consideration.
Preparation and sterilization of media
According to the specific industrial production basic components needed to carry out fermentation are selected as per the required volume.
Medium components should be free from contamination. So all the medium components employed in the fermentation process are sterilized. Sterilization is mostly carried out by applying heat and to lesser extent other physical methods, chemical methods (disinfectants) and radiation (using UV rays, γ rays). Batch Sterilization is carried out at 121°C (20 to 60 mins) where as continuous sterilization is done at 140°C for (30 to 120 secs).
Much energy is wasted on batch sterilization on compared with continuous sterilization nearly 80 to 90% of energy saved during this process. Air and heat sensitive components are sterilizied by membrane filters.
Fermentation Process
It involves the propagation of the microorganism and the production of the desired product. Fermentation process is divided depending on the feeding strategy of the culture and medium as follows.
- Batch Fermentation
- Continuous Fermentation
- Fed batch Fermentation
1. Batch Fermentation
The medium and culture are initially fed into the vessel and it is then closed. After that, no components are added apart from Oxygen. The pH is adjusted during the course of process by adding either acid or alkali. The fermentation is allowed to run for a predetermined period of time and the product is harvested at the end.
Foaming is controlled by adding antifoam agents such as palm oil or soybeans oil. Heat generated is regulated by providing water circulation system around the vessel for heat exchange.
2. Continuous fermentation
This is an open system. It involves the removal of culture medium continuously and replacement of them with a fresh sterile medium in a bioreactor. In this method, homogenously mixing reactors which include chemo stat and turbid stat bioreactors are used. Examples: production of antibiotics, organic solvents,
beer, ethanol and SCP.
3. Fed batch system
It is a combination of both batch and continuous systems. In this, additional nutrients are added to the fermentors as the fermentation is in progress. This extends the time of operation, but the products are harvested at the end of the production cycle as in batch fermenter. Followed by the fermentation, production, products are harvested or separated by downstream processing.
Downstream Processing
The various processes used for the actual recovery of useful products from fermentation or any other industrial processes are called downstream processing. The cost of downstream processing (DSP) is often more than 50% of the manufacturing cost, and there is product loss at each step of DSP.
Therefore, the DSP should be efficient, involve as few steps as possible and be cost-effective. Methods involved in the downstream processing are outlined in the flowchart (6.2). Table 6.4 shows Difference between upstream and downstream processing.
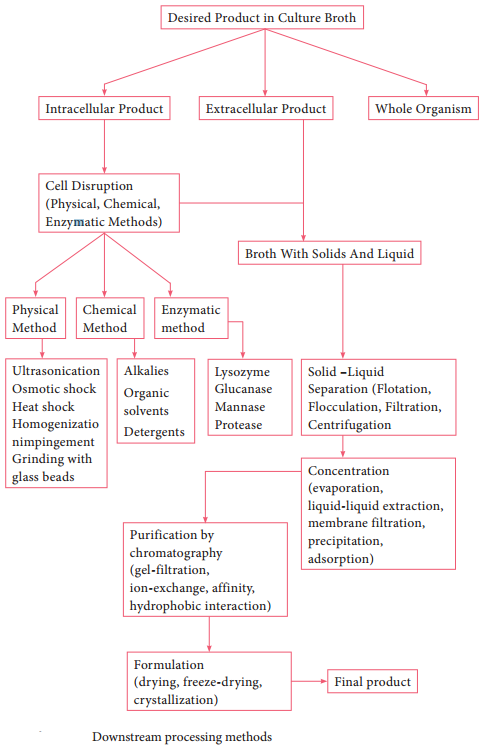
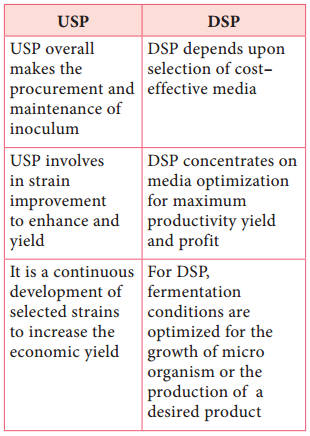
Table 6.4: Diffrence between upstream (usp) and downstream (dsp) processing