Learninsta presents the core concepts of Biology with high-quality research papers and topical review articles.
Gene As The Functional Unit Of Inheritance
A gene is a basic physical and functional unit of heredity. The concept of the gene was first explained by Gregor Mendel in 1860’s. He never used the term ‘gene’. He called it ‘factor’. In 1909, the Danish biologist Wilhelm Johannsen, coined the term ‘gene’, that was referred to discrete determiners of inherited characteristics.
According to the classical concept of gene introduced by Sutton in 1902, genes have been defined as discrete particles that follow Mendelian rules of inheritance, occupy a definite locus in the chromosome and are responsible for the expression of specific phenotypic character. They show the following properties:
- Number of genes in each organism is more than the number of chromosomes; hence several genes are located on the same chromosome.
- The genes are arranged in a single linear order like beads on a string.
- Each gene occupies a specific position called locus.
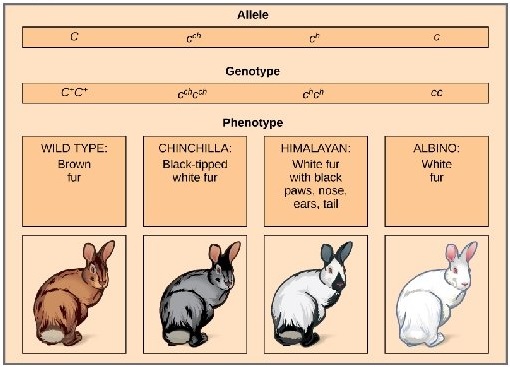
- Genes may exist in several alternate forms called alleles.
- Genes may undergo sudden change in positions and composition called mutations.
- Genes are capable of self-duplication producing their own copies.