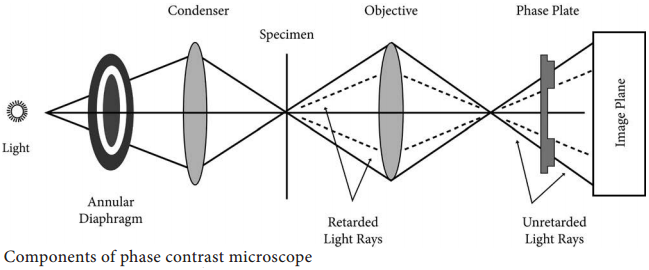
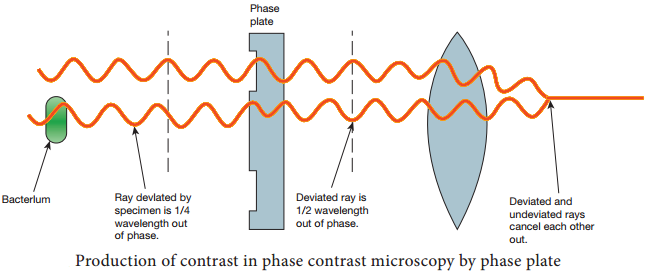
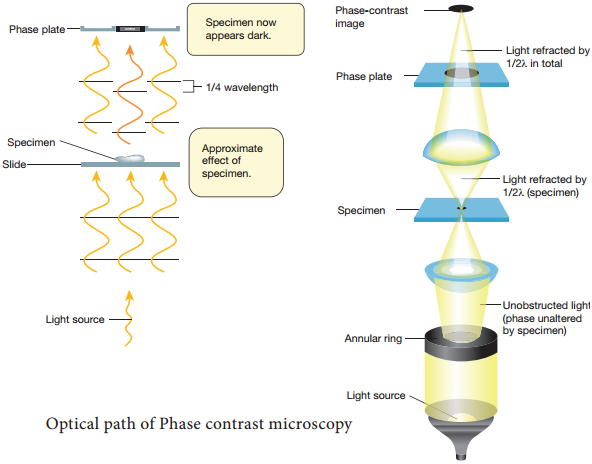
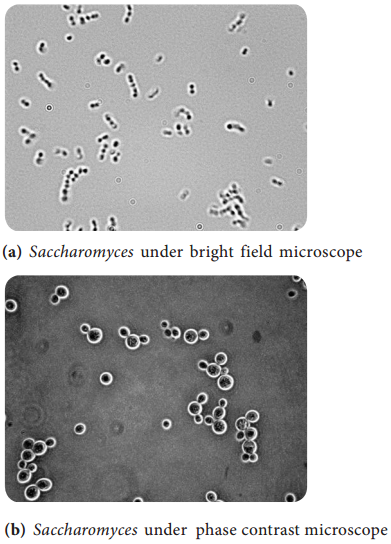
Learninsta presents the core concepts of Microbiology with high-quality research papers and topical review articles.
Electron Microscope – Definition, Principle, Parts, Uses
Examining the ultra structure of cellular components such as nucleus, plasma membrane, mitochondria and others requires 10,000X plus magnification which was just not possible using Light Microscopes. This is achieved by Electron microscopes which have greater resolving power than light microscopes and can obtain higher magnifications.
In an electron microscope, a focused electron beam is used instead of light to examine objects. Electrons are considered as radiation with wavelength in the range 0.001 – 0.01 nm compared to 400 – 700 nm wavelength of visible light used in an optical microscope.
Optical microscopes have a maximum magnification power of 1000X, and resolution of 0.2 μm compared to resolving power of the electron microscope that can reach 1,000,000 times and resolution of 0.2 nm. Hence, electron microscopes deliver a more detailed and clear image compared to optical microscopes. Table 2.1 differentiate electron microscope from light microscope.
| Light Microscope | Electron Microscope |
| 1. Light is the illuminating source | 1. The beam of electrons is the electron source |
| 2. Specimen preparation takes usually few minutes to hours. Live or dead specimen may be seen. | 2. Specimen preparation takes usally takes a few days. Only dead or dried specimen are seen. |
| 3. Condenser, objective and eye piece lenses are made up of glasses. | 3. All lenses are electromagnetic. |
| 4. Specimen is stained by coloured dyes. | 4. Specimen is coated with heavy metals in order to reflect electrons. |
| 5. It has low resolving power (0.25 pm to 0.3 pm). It has a magnification of 500X to 1500X. | 5. It has high resolving power (0.001pm), about 250 times higher than light microscope. It has a magnification more than 100,000X |
| 6. Vaccum is not required | 6. Vaccum is essential for its operation |
| 7. Image is seen by eyes through ocular lens | 7. Image is produced on fluorescent screen or photographic plate. |
Types of Electron Microscopes
- Transmission electron microscopes (TEM)
- Scanning electron microscopes (SEM)
- Scanning transmission electron microscopes (STEM)
The electron microscope was invented in 1931 by two German scientists, Ernst Ruska and Max Knoll. Ernst Ruska later received Nobel Prize for his work in 1986. The Transmission Electron Microscope (TEM) was the first type of Electron Microscope to be developed.
Principle
The fundamental principle of electron microscope is similar to light microscope. In electron microscope, a high velocity beam of electrons is used instead of photons. In the electron gun, electrons are emitted from the surface of the cathode and accelerated towards the anode by high voltage to form a high energy electron beam.
All lenses in the electron microscope are electromagnetic. Charged electrons interact with the magnetic fields and magnetic force focuses an electron beam. The condenser lens system controls the beam diameter and convergence angles of the beam incident on a specimen.
The image is formed either by using the transmitted beam or by using the diffracted beam. The image is magnified and focused onto an imaging device, such as a fluorescent screen, on a layer of photographic film, or to be detected by a sensor.
Sample Preparation:-
Preparation of specimens is the most complicated and skillful step in EM. The material to be studied under electron microscopy must be well preserved, fixed, completely dehydrated, ultrathin and impregnated with heavy metals that sharpen the difference among various organelles.
The material is preserved by fixation with glutaraldehyde and then with osmium tetroxide. The fixed material is dehydrated and then embedded in plastic (epoxy resin) and sectioned with the help of diamond or glass razor of ultra-microtome.
In TEM, sample sections are ultrathin about 50 – 100 nm thick. These sections are placed on a copper grid and exposed to electron dense materials like lead acetate, uranylacetate, phosphotungstate. In SEM, samples can be directly imaged by mounting them on an aluminum stub.
Electron – Sample Interactions:-
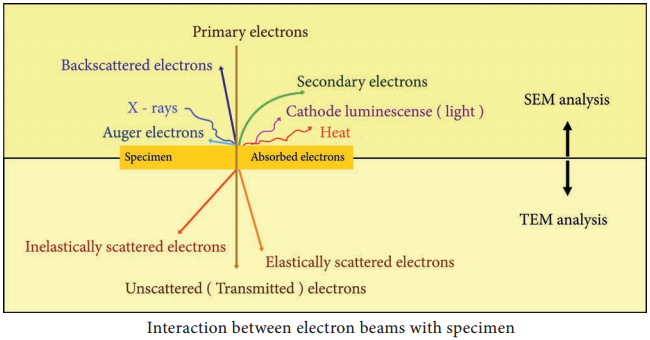
Interaction of electron beam with the sample results in different types of electrons: Elastic scattered electrons, Inelastic scattered electrons, secondary electrons and backscattered electrons. Almost all types of electron interactions can be used to retrieve information about the specimen.
Depending on the kind of radiation or emitted electrons which are used for detection, different properties of the specimen such as topography, elemental composition can be concluded. Figure 2.8 shows the interaction of the electron beam with the specimen.
In Transmission electron microscope (TEM), a beam of electrons is transmitted through an ultrathin specimen, interacting with the specimen as it passes through. An image is formed from the interaction of the transmitted unscattered electrons through the specimen.
Secondary electrons are mainly used in scanning electron microscope (SEM) for imaging the surface topography of biological specimens. The interaction of electron beam with samples results in secondary electrons and backscattered electrons that are detected by standard SEM equipment.
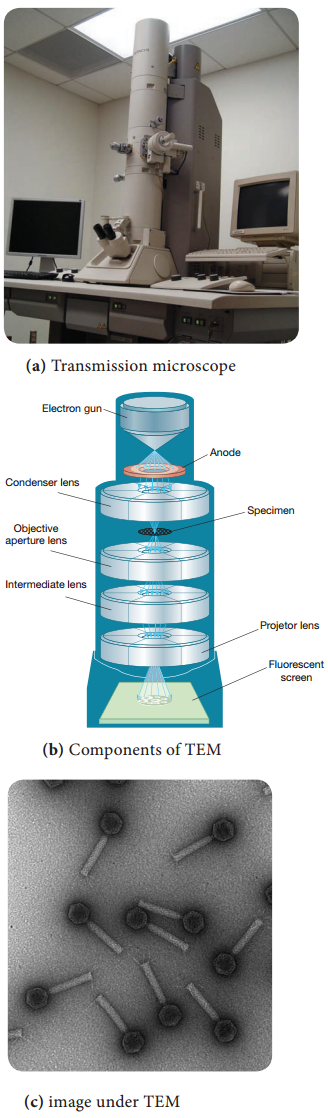
Working Principle and Instrumentation of TEM
The optics of the TEM is similar to conventional transmission light microscope. A transmission electron microscope has the following components,
- Electron gun
- Condenser lens
- Specimen stage
- Objective lens and projector lens
- Screen/photographic film/Charged Coupled Device (CCD) camera
Electron Gun consists of a tungsten filament or cathode that emits electrons on receiving high voltage electric current (50,000 – 100,000 volts). A high voltage between the electron source (cathode) and an anode plate is applied leading to an electrostatic field through which the electrons are accelerated.
The emitted electrons travel through vacuum in the microscope column. Vacuum is essential to prevent strong scattering of electrons by gases. Electromagnetic condenser lenses focus the electrons into a very thin beam. Electron beam then travels through the specimen and then through the electromagnetic objective
lenses.
In a TEM microscope, the sample is located in the middle of the column. At the bottom of the microscope, unscattered electrons hit the fluorescent screen giving image of specimen with its different parts displayed in varied darkness, according to their density. The image can be studied directly, photographed or digitally
recorded. Figure 2.9 show the arrangement of components for transmission electron microscope.
Information that can be obtained using TEM include,
- Topography: surface features, texture
- Morphology: shape and size of the particles
- Crystallographic arrangement of atoms
- Composition: elements and the their relative amounts.
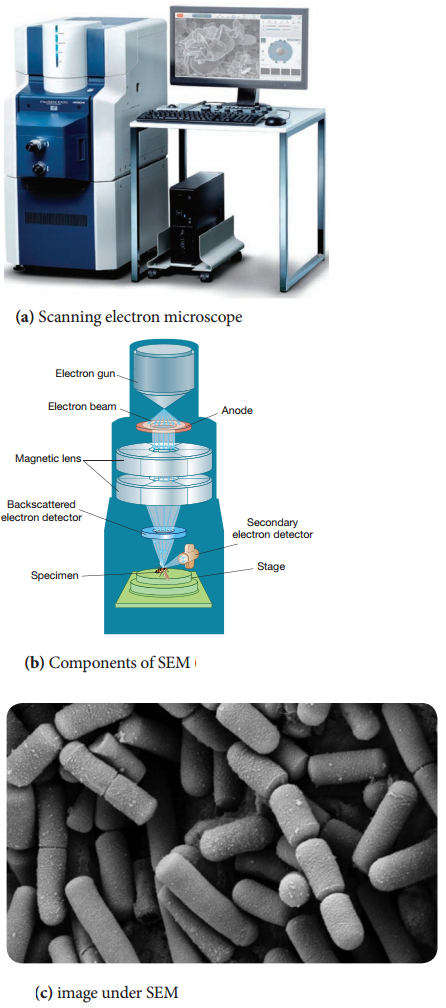
Working Principle and Instrumentation of SEM
It is first built by Knoll in 1935. It is used to study the three dimensional images of the surfaces of cells, tissues or particles. The SEM allows viewing the surfaces of specimens without sectioning. The specimen is first fixed in liquid propane at-180°C and then dehydrated in alcohol at-70°C.
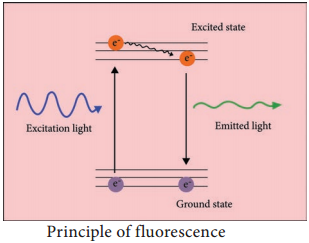
The dried specimen is then coated with a thin film of heavy metal, such as platinum or gold, by evaporation in a vacuum provides a reflecting surface of electrons. In SEMs, samples are positioned at the bottom of the electron column and the scattered electrons (backscattered or secondary) are captured by electron detectors.
In SEM, there are several electromagnetic lenses, including condenser lenses and one objective lens. Electromagnetic lenses are for electron probe formation, not for image formation directly, as in TEM. Two condenser lenses reduce the crossover diameter of the electron beam. The objective lens further reduces the cross-section of the electron beam and focuses the electron beam as probe on the specimen surface (Figure 2.10).
Objective lens thus functions like a condenser. This is in contrast to TEM where objective lens does the magnification. Major difference between SEM and TEM are given in Table 2.2. SEMs are equipped with an energy dispersive spectrometer (EDS) detection system which is able to detect and display most of the X-ray spectrum. The detector normally consists of semiconducting silicon or germanium. Difference between SEM and TEM.
|
Properties |
SEM |
TEM |
| 1. Types of electrons | It is based on scattered electrons that are emitted from the surface of a specimen | It is based on transmitted electrons |
| 2. Sample preparation | Sample can be of any thickness and is coated with a thin layer of a heavy metal such as gold or palladium and mounted on an aluminium slab | Laborious sample preparation is required. The sample has to be cut into thin sections so as to allow electrons to pass through it and are supported on TEM grids. |
| 3. Resolution | The resolution is up to 20nm | TEM has much higher resolution than SEM. It can resolve objects as close as 1nm |
| 4. Magnification | The magnifying power of SEM is up to 100,000X | The magnifying power of TEM is up to 5,000,000 X |
| 5. Image formation | SEM provides a 3 dimensional image. Secondary or back scattered electrons are captured, detected and displayed on computer screen | TEM provides a 2 dimensional image. Transmitted electrons hit a fluroscent screen giving rise to a shadow image. The image can be studied directly by the operator or photographed with a camera. |
| 6. Application | SEM is used to study the topography and atomic composition of specimens | TEM is used to study the interior of cells, the structure of protein molecule, the organization of molecules in viruses and cytoskeletal filaments and the arrangement of protein molecules in cell membranes. |
Scanning transmission electron microscopy (STEM) combines the principles of transmission electron microscopy and scanning electron microscopy and can be performed on either type of instrument. Like TEM, STEM requires very thin samples and the primary electron beam is transmitted by the sample.
One of its principal advantages over TEM is in enabling the use of other of signals that cannot be spatially correlated in TEM, including secondary electrons, scattered beam electrons, characteristic X-rays, and electron energy loss.