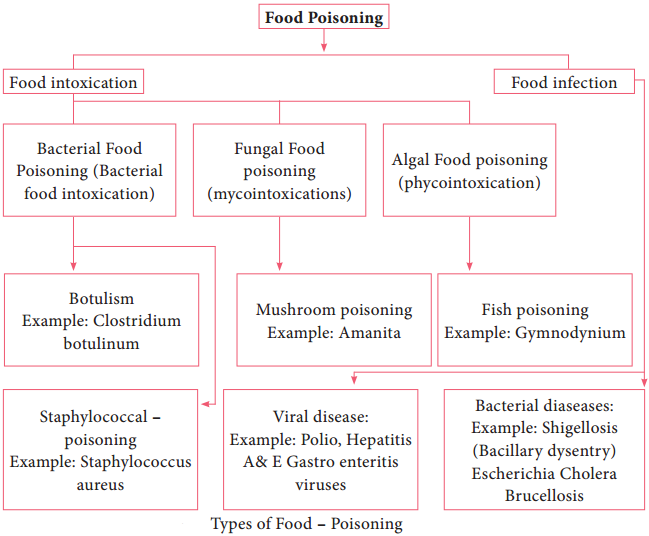
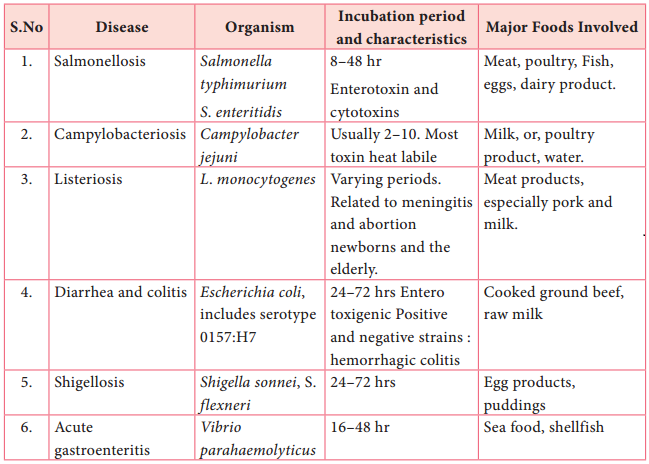
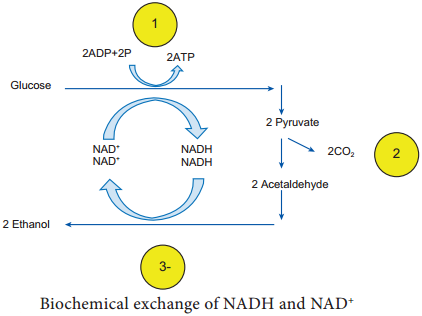
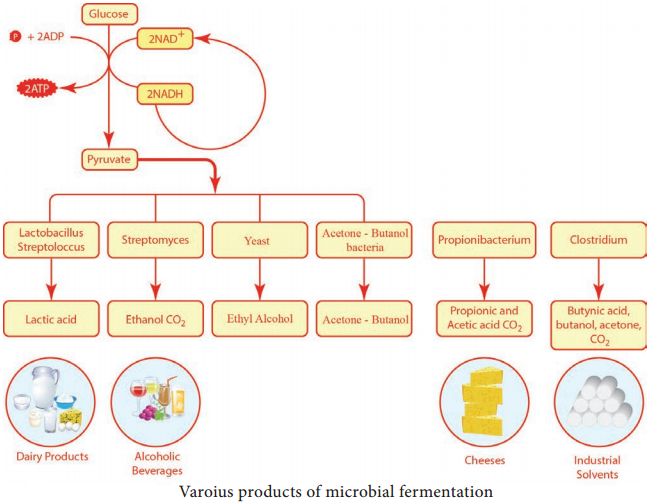
Learninsta presents the core concepts of Microbiology with high-quality research papers and topical review articles.
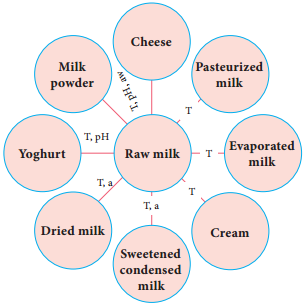
Food Microbiology of Cheese
There are about 2000 varieties of cheese made from mammalian milk. Cheese is thought to have originated in south western Asia some 8000 years ago. The Romans encouraged technical improvements and stimulated the development of new varieties during their invasion in Europe between 60 B.C and A.D. 300. The cheese name is derived from Latin name caseus (Figure 5.5).

They are two groups of cheese, fresh cheese and ripened cheese. The fresh cheese are made up of milk coagulated by acid or high heat. Example: cottage cheese. Ripened cheese are made through lactic acid bacterial fermentation and coagulated by an enzyme preparation. The curd is removed and salted and whey
is separated. The salted curd is held in controlled environment.
During this process, various physical and chemical changes occur to give a characteristic flavour and texture. So the mammalian origin of milk influences the flavour and aroma of a natural ripened cheese.
Microbiology of cheese
A large number of microorganisms plays a role in the ripening process. On the first day of cheese making process, the microbial number in the starting material ranges from one to two billion. Therefore, the production declines because of insufficient oxygen, high acidity and the presence of inhibitory
compounds that are produced as the cheese ripens.
It is mainly the action of their cellular enzymes on lactose, fat and proteins that creates the ripened cheese flavour. The gas forming culture of Propionibacterium shermanii is essential for giving swiss cheese its eye, or holes and flavour (Figure 5.6).

The specificity of cheese depends upon the varieties of microorganisms used. The process of cheese making, involves nine steps:
a. Preparing the milk
b. Forming a curd.
c. Cutting
d. Cooking
e. Separating the whey
f. Salting the residue
g. Applying microbes
h. Pressing the curd
i. Ripening the young cheese
Types of Cheese
Cheese can be divided among different categories or types, according to their firmness. There are various system for classifying cheese and there are variations within each system (Table 5.6).
Types of Cheese