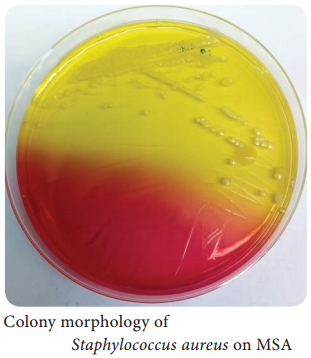
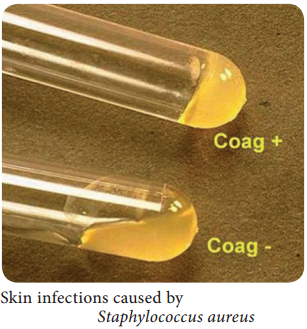
Learninsta presents the core concepts of Microbiology with high-quality research papers and topical review articles.
Vibrio Cholerae
Vibrio is one of the curved rod bacteria, prominent in the Medical Bacteriology. They are present in marine environment and surface waters worldwide.
Vibrio is a member of the family Vibrionaceae. The most important member of this genus is Vibrio cholerae, the causative agent of cholera. The term Vibrio is derived from Vibrare (Latin word) which means “to shake or vibrate” and the word Cholera is derived from Chole (Greek word) which means, “to bile”.
Morphology
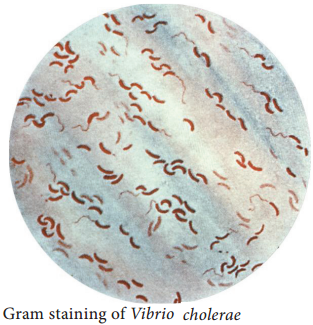
Vibrio cholerae is gram negative, curved or comma shaped, (1.5um × 0.2 – 0.4um in size) non – capsulated. The organism is very actively motile with a single polar flagellum and the characteristic movement is called as darting motility. In stained smears of mucus flakes from acute cholera patients, the Vibriois seen
arranged in parallel rows. This was described by Robert Koch as “fish in stream” appearance.
Culture Characteristics
Vibrio cholera is strongly aerobic. It grows best in alkaline media with the optimum temperature 37°C and pH 8.2. It is nonhalophilic, therefore, cannot grow in media with a concentration of sodium chloride more than 7% (Figure 7.17). Some of the media in which Vibrio cholerae are cultivated are tabulated below
in Table 7.16.
Table 7.16: Colony morphology of Vibrio cholerae on various media
|
Media |
Colony morphology |
| Nutrient agar | The colonies are moist, translucent round disks (1-2mm in diameter) with a bluish tinge in transmitted light. |
| MacConkey agar | The colonies are colorless at first but become reddish on prolonged incubation due to late fermentation of lactose. |
| Thiosulphate citrate bile sucrose sugar (pH 8.6) | It is used as a selective medium for isolation of Vibrios. It produces large yellow convex colonies due to sucrose fermentation. |
Enterotoxin
Vibrios multiplying on the intestinal epithelium produce an enterotoxin called Cholera toxin. It is also known as Choleragen (or CT). This toxin molecule is approximately 84,000 Dalton and consists of two major subunits namely A and B There is only one subunit in A (1A) whereas there are five subunits in B (5B) (Figure 7.18).
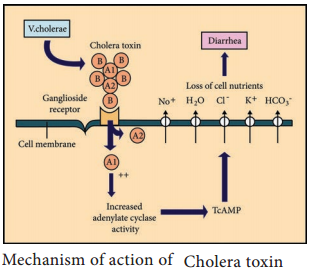
Mode of Action
- The B (binding) units of enterotoxin get attached to the GM1 (Ganglioside membrane receptors I) on the surface of jejunal epithelial cells. (target cells).
- The A (active) subunits then enters the target cell and dissociates into 2 fragments, A1 & A2. The A2 fragment links biologically active A1 fragment to the B – subunit.
- The A1 fragment causes prolonged activation of cellular adenylate cyclase which in turn accumulates CAMP in the target cell. This leads to outpouring of large quantities of water and electrolytes into small intestinal lumen. Thus, resulting in profuse watery diarrhea.
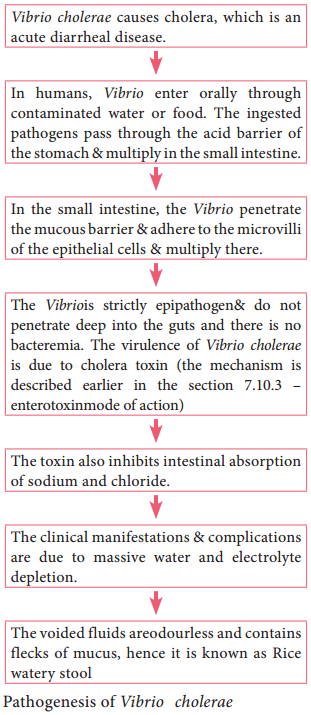
Pathogenesis
The pathogenic mechanism of Vibrio choleraeis discussed below in flowchart 7.7.
Source of Infection – contaminated water or food
Route of entry – fecal – oral route
Site of infection – small intestine
Incubation period – few hours to 5 days (usually 2 – 3 days)
Clinical Feature
Dehydration, anuria (absence of urine excretion), muscle cramps, hypokalemia (low blood potassium) & metabolic acidosis (low serum concentration of bicarbonates).
Laboratory Diagnosis
Specimen: Stool
Direct microscopy:
It is not a reliable method for rapid diagnosis, the characteristic darting motility of the vibrio can be observed under dark – field microscope.
Culture:
Stool sample is directly inoculated on MacConkey agar and TCBS agar. The plates are examined after overnight incubation at 37°C for typical colonies of Vibrio cholera, and the colonies are identified by gram staining and oxidasetest.
Prophylaxis
1. General Measures:
- Purification of water supplies
- Improvement of environment sanitation
- Infected patients should be isolated, and their excreta must be disinfected
2. Vaccines:
Two types of oral vaccines have been tried recently:
- Killed oral whole cell vaccines
- Live oral vaccines
Treatment
1. Oral Rehydrationtherapy:
The severe dehydration & salt depletion can be treated by oral rehydration therapy (as recommended by WHO).
2. Antibiotics:
It is of secondary importance, oral tetracycline was recommended for reducing the period of Vibrio excretion.