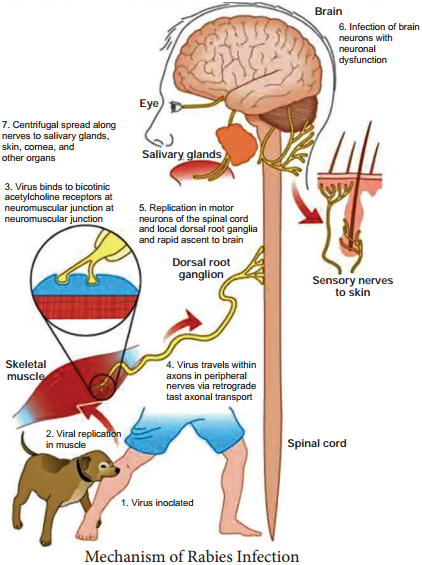
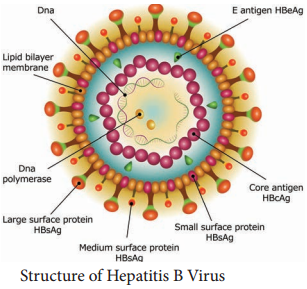
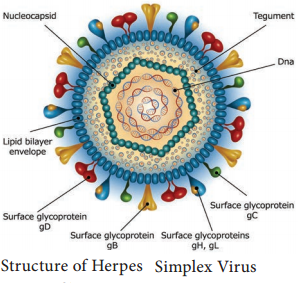
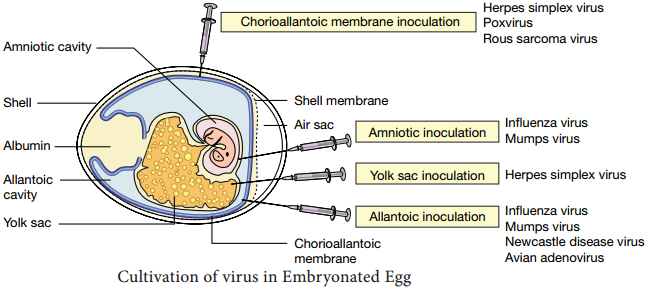
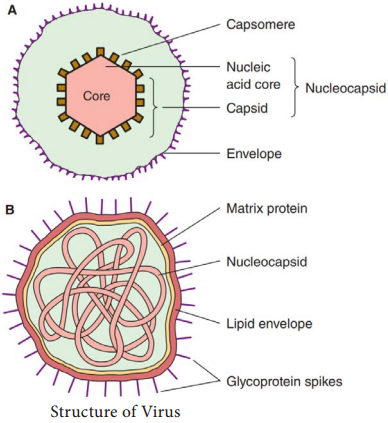
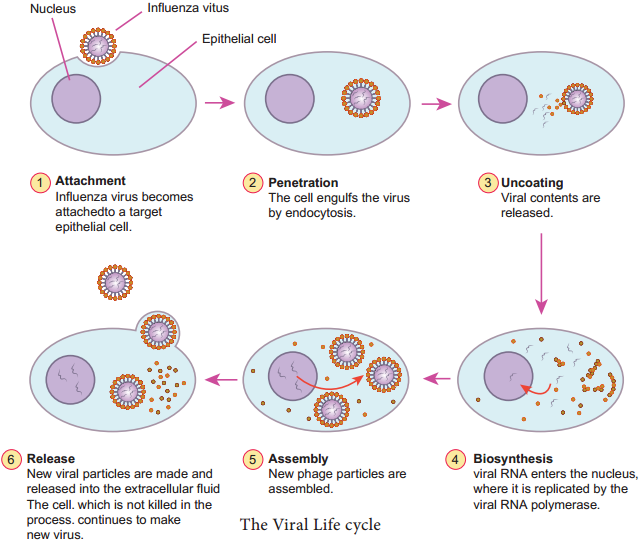
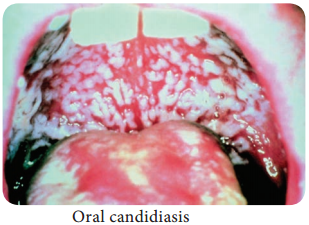
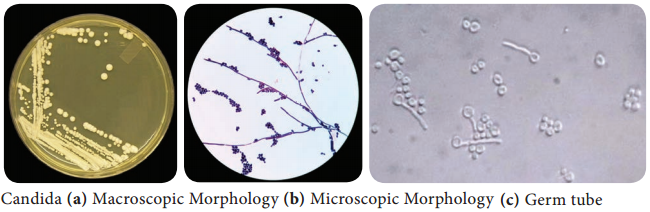
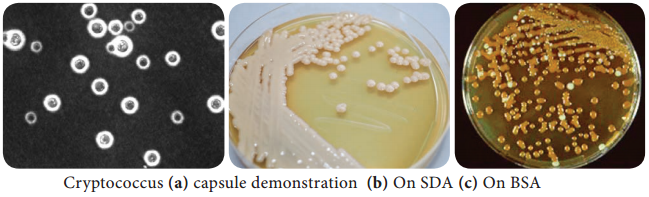
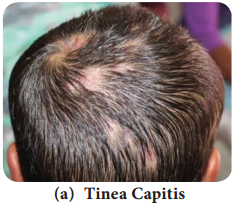
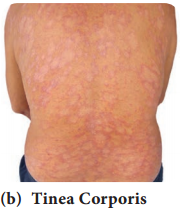
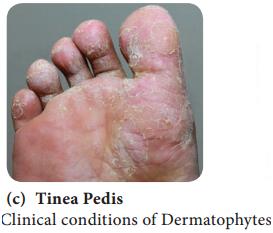
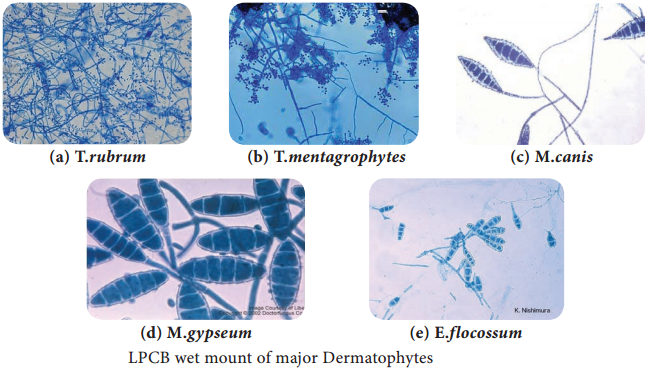
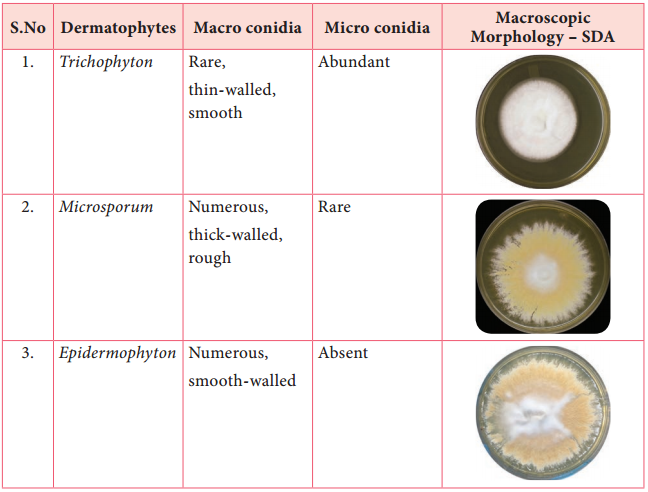
Learninsta presents the core concepts of Microbiology with high-quality research papers and topical review articles.
Antigen Antibody Reactions and its types | Working principle, Applications
The interaction between antigen and antibody is called antigen-antibody reactions. It is abbreviated as Ag-Ab reaction. This reaction is the basis of humoral immunity. The antigen and the antibody react to form immune complex.
Ag + Ab …………….. Ag – Ab complex The reaction between antigen and antibody is highly specific. It is compared to the lock and key system. The part of the antigen that combines with the antibody is called epitope or antigenic determinant. The part of antibody which combines with the antigen is called paratope or antigen determining site. Most of the antibodies have two binding sites and IgM has 5-10 binding sites.
Immunofluorescence
When antibodies are mixed with the fluorescent dyes such as fluorescein or rhodamine, they emit radiation. This phenomenon of emitting radiation by antibodies labelled with fluorescent dye is called immuno fluorescence. This reaction is well observed under fluorescent microscope. It is used to locate and identify antigens in tissues.
Types of Immunofluorescence
- Direct method
- Indirect method
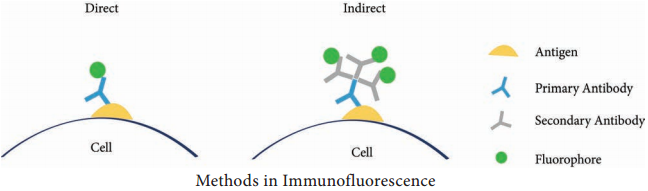
Direct Method
In this method, the antibody labelled with fluorescent dye is directly applied on the tissue section. The labelled antibody binds with specific antigen. This can be observed under the fluorescent microscope.
Indirect Method
In this method, unlabelled antibodies are directly applied on the tissue sectionswhich bind with the specific antigens. Then the antibody labelled with the fluorescent dye is added to the tissue. Anti-antibody specifically binds with already added or linked unlabelled antibody (Figure 11.1).
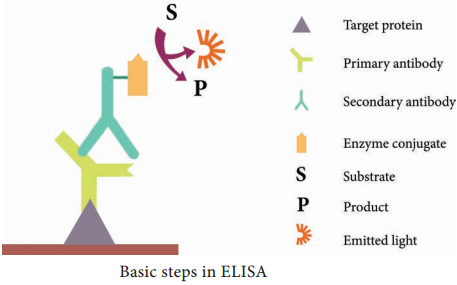
ELISA (Enzyme Linked Immuno Sorbent Assay)
ELISA (Enzyme-Linked Immuno Sorbent Assay) is a plate-based assay technique designed for detecting and quantifying substances such as peptides, proteins, antibodies and hormones. It is also known as Enzyme Immuno Assay (EIA).
In 1971, after the descriptions of Peter Perlmann and Eva Engvall at Stockholm University in Sweden, ELISA has become the system of choice when assaying soluble antigens and antibodies. All assays for antibody production depend upon the measurement of interaction of elicited antibody with antigen.
Principle
The principle of ELISA is very simple. The test is generally conducted in micro titre plates. (Figure 11.2 Micro titre plate).

If the antigen is to be detected the antibody is fixed in the micro titre plate and vice versa. Test sample is added in the microtitre plate, if there is presence of Ag or Ab in the test sample, there will be Ag-Ab reactions (with immobilized Ab or Ag). Later enzyme labelled antibody is added in the reaction mixture, which will combine with either test antigen or Fc portion of test antibody.
The enzyme system consists of:
1. An enzyme:
Horse Radish Peroxidase(HRP), alkaline phosphatase which is labelled or linked, to a specific antibody.
2. A specific substrate:
- O-Phenyl-diaminedihydrochloride for peroxidase
- P nitrophenyl Phosphate – for Alkaline Phosphatase
Substrate is added after the antigenantibody reaction. The enzyme hydrolyses the substrate to give a yellow colour compound in case of alkaline phosphatase (Figure 11.3). The intensity of the colour is proportional to the amount of antibody or antigen present in the test sample, which can be quantified using ELISA reader
(Figure 11.4 ELISA reader)
Types
There are four kinds of ELISA assay tests. They are: Direct ELISA, Indirect ELISA, Sandwich ELISA and Competitive ELISA (Figure 11.5).
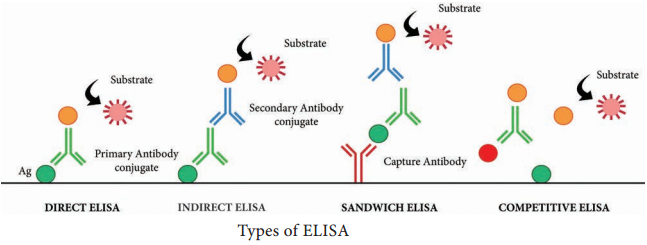
i. Direct ELISA
An antigen is immobilized in the well of an ELISA plate. The antigen is then detected by an antibody directly conjugated to an enzyme such as HRP. Direct ELISA detection is much faster than other ELISA techniques as fewer steps are required.
The assay is also less prone to error since fewer reagents and steps are needed, i.e. no potentially cross-reacting secondary antibody needed. Finally, the direct ELISA technique is typically used when the immune response to an antigen needs to be analyzed.
ii. Indirect ELISA
Indirect ELISA is used to detect antibody. A known antigen is coated on the micro titre plate. If the patient’s serum contains antibody specific to the antigen, the antibody will bind to the antigen.
After incubation the wells are washed and the enzyme labelled anti Human Gamma Globulin (HGG) is added to the well. AntiHGG can react with antigen antibody complex. The substrate for the enzyme is added finally which is hydrolysed by the enzyme which develops a colour.
iii. Sandwich ELISA
Sandwich ELISA is used to detect antigen. A known antibody is coated on the micro titre plate. A test antigen is added to each well and allowed to react with the bound antibody. If the patient’s serum contains antigen specific to the antibody, the antigen will bind to the antibody.
Specifically bound antigen and antibody will remain in the wells even after washing. The second antibody is added and allowed to react with bound antigen. Substrate is added to measure colour reaction.
iv. Competitive ELISA
It is used for the detection of antigens. Antibody is first incubated with a sample-containing antigen. The antigen and antibody complex is added to the antigen coated microtitre well. If more antigen present in the sample, the less free antibody will be available to bind to the antigen coated well.
Addition of an enzyme conjugated secondary antibody specific to the primary antibody can be used to determine the amount of primary antibody bound to the well. It is a quantitative test for the antigen detection.
Application
An ELISA test may be used to diagnose:
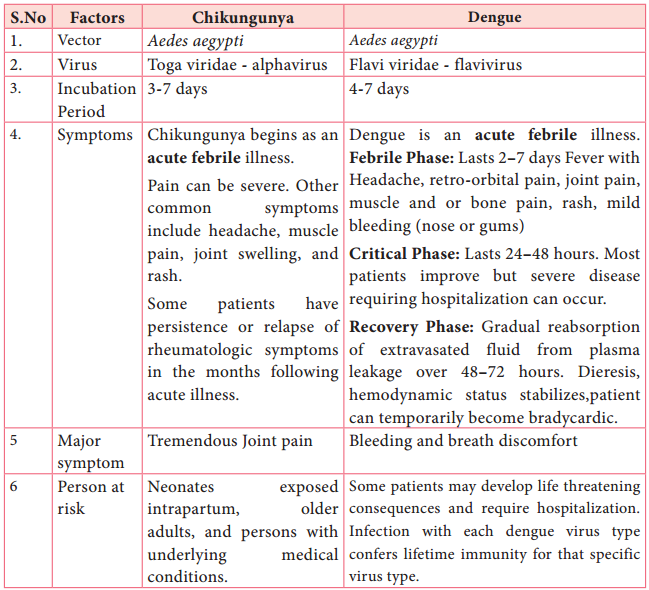
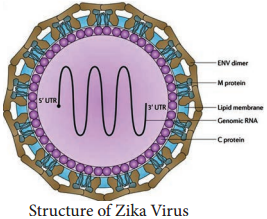
HIV, Lyme disease, pernicious anaemia, Rocky Mountain spotted fever, rotavirus, squamous cell carcinoma, syphilis, toxoplasmosis, varicella-zoster virus, which causes chickenpox and Zika virus.