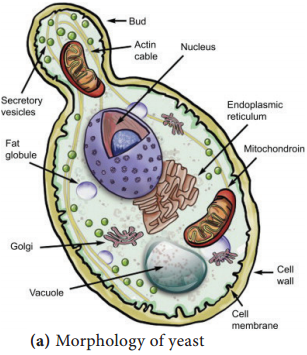
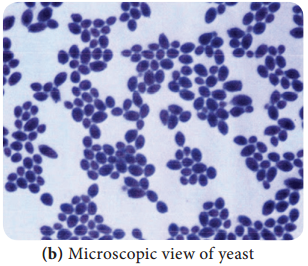
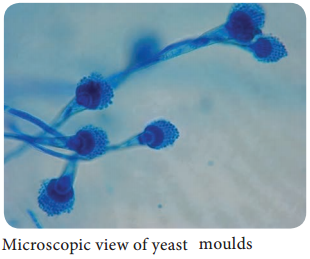
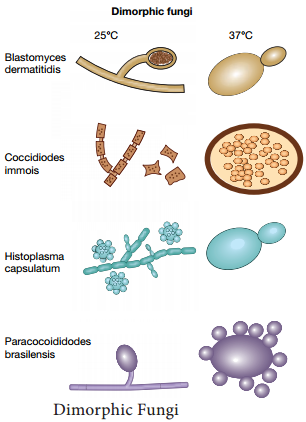
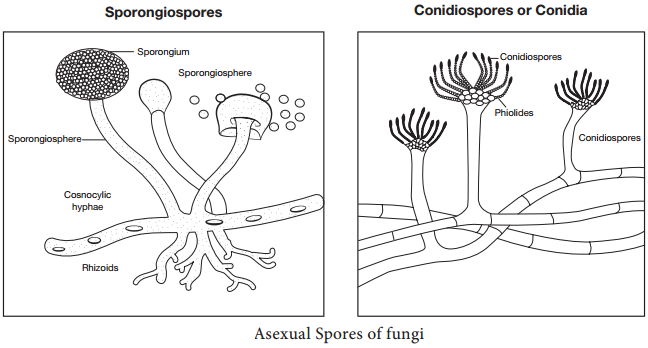
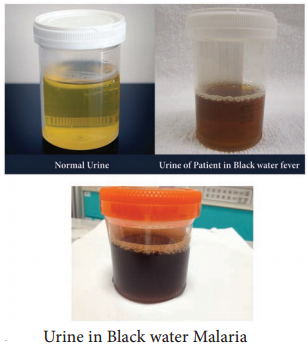
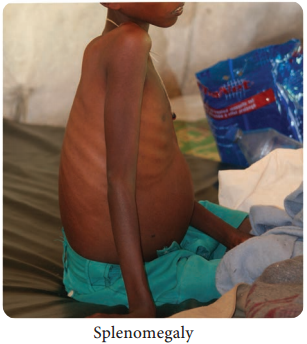
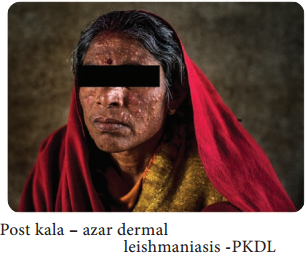
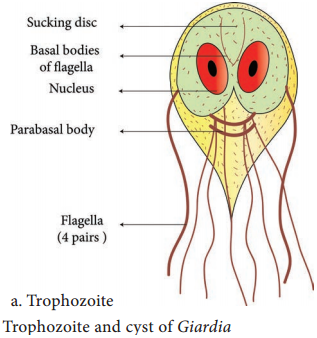
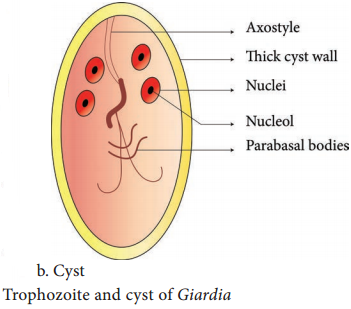
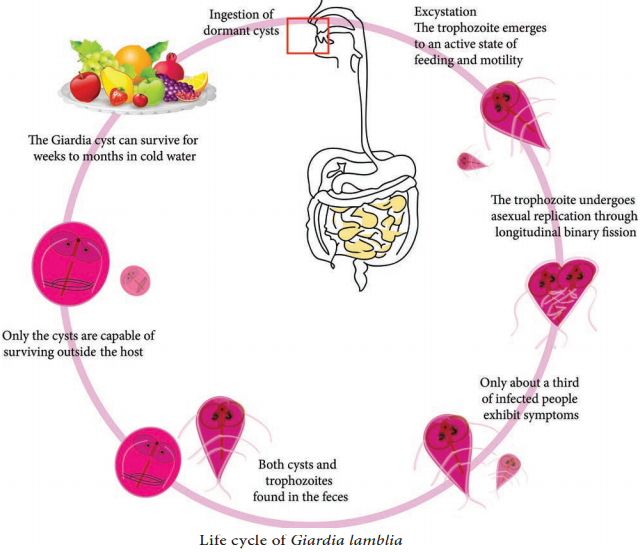
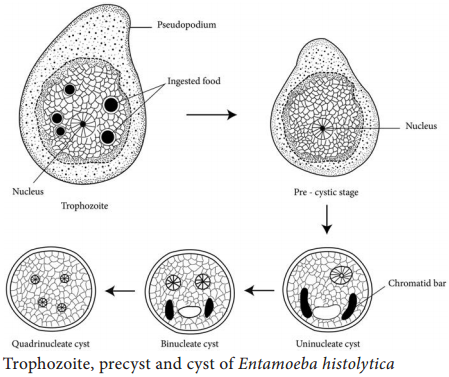
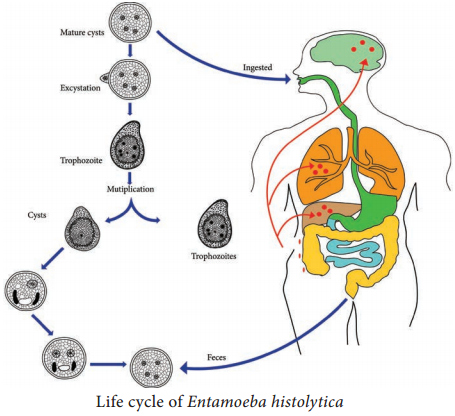
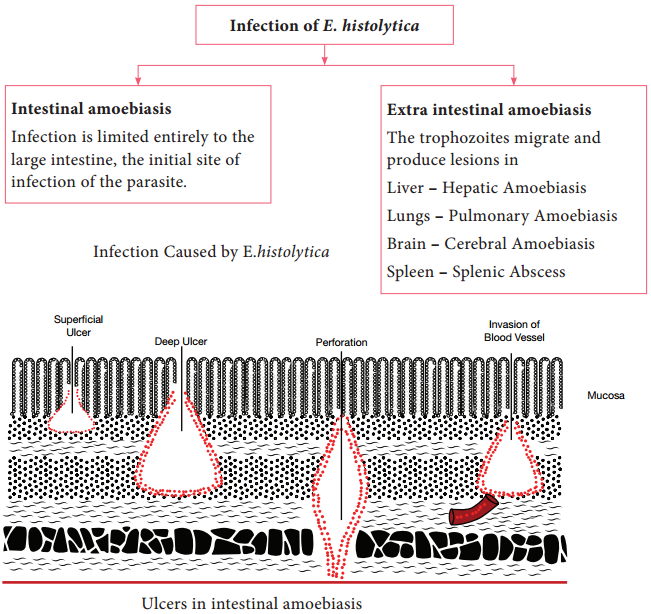
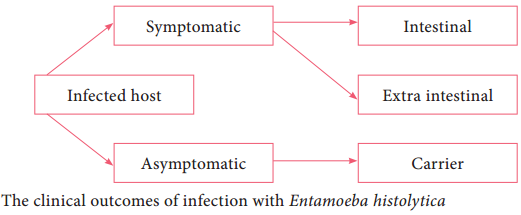
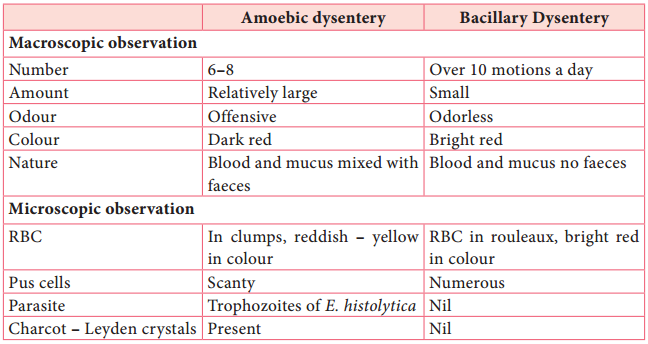
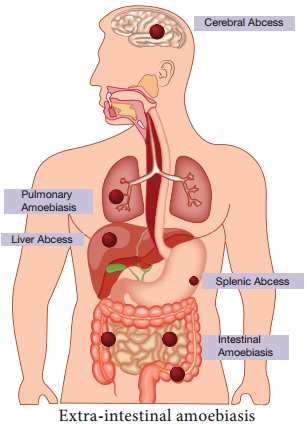
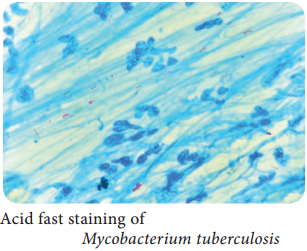
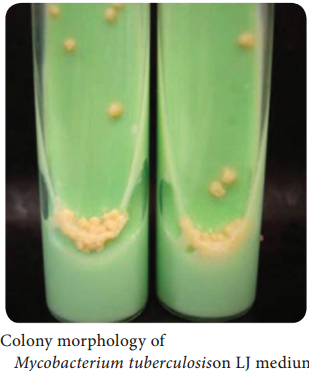
Learninsta presents the core concepts of Microbiology with high-quality research papers and topical review articles.
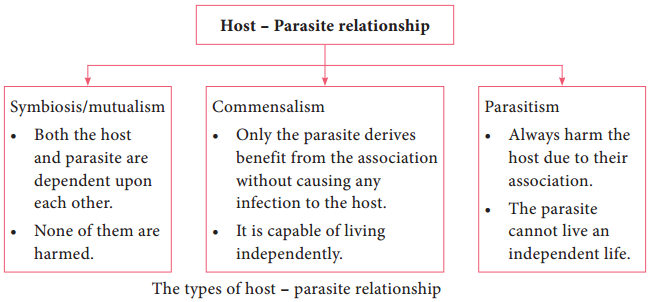
Superficial Cutaneous Mycosis
The superficial cutaneous fungal infections involve the outer most layers of skin and its appendages like hair and nails. The causative agents colonize on epidermis or supra – follicular portions of hair and do not penetrate into deeper layers.
The genus Malassezia is responsible for the superficial infection of the skin. Malassezia furfur is lipophilic yeast. It is a commensal of normal skin in the sebaceous glands of warm – blooded vertebrates.
It may be pathogenic under certain conditions usually causing skin conditions like Pityriasis versicolor, Seborrheic dermatitis, Atopic dermatitis, Malassezia folliculitis and systemic infection. Symptoms include macular, erythematous, hyper pigmented or hypo pigmented lesions with fine scaling.
Tinea nigra is responsible for the superficial cutaneous infection of the skin. Hortaea werneckii is the phaeoid (dematiaceous) fungi causes infection on the palms and soles. It is also commonly termed as Tinea nigra palmaris and Tinea nigra plantaris. Symptoms includes brown to black deeply pigmented non – scaly, macular lesions affecting skin of the palms and occasionally soles.
Piedra causes superficial infection of hair shaft. The word Piedra is derived from Spanish word Stone. There are two types of Piedra based on causative fungi and characteristics of nodules. They are Black piedra caused by Piedraia hortae and White piedra caused by Trichosporon species.
The symptoms include development of firm, irregular nodules of fungal elements cemented to the hair. The piedra can be distinguished on the basis of shape, size and pigmentation of fungal cells of nodules which are found around hair cortex.